New Class of Benzodiazepinone Derivatives as Pro-Death Agents Targeting BIR Domains in Cancer Cells
- PMID: 36615638
- PMCID: PMC9823934
- DOI: 10.3390/molecules28010446
New Class of Benzodiazepinone Derivatives as Pro-Death Agents Targeting BIR Domains in Cancer Cells
Abstract
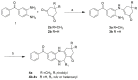
Inhibitor of Apoptosis Proteins (IAPs) are validated targets for cancer therapy, and the deregulation of their activities within the NF-κB pathway correlates with chemoresistance events, even after treatment with IAPs-antagonists in the clinic (Smac-mimetics). The molecule FC2 was identified as a NF-κB pathway modulator in MDA-MB-231 adenocarcinoma cancer cells after virtual screening of the Chembridge library against the Baculoviral IAP Repeat 1 (BIR1) domain of cIAP2 and XIAP. An improved cytotoxic effect is observed when FC2 is combined with Smac-mimetics or with the cytokine Tumor Necrosis Factor (TNF). Here, we propose a library of 22 derivatives of FC2, whose scaffold was rationally modified starting from the position identified as R1. The cytotoxic effect of FC2 derivatives was evaluated in MDA-MB-231 and binding to the cIAP2- and XIAP-BIR1 domains was assessed in fluorescence-based techniques and virtual docking. Among 22 derivatives, 4m and 4p display improved efficacy/potency in MDA-MB-231 cells and low micromolar binding affinity vs the target proteins. Two additional candidates (4b and 4u) display promising cytotoxic effects in combination with TNF, suggesting the connection between this class of molecules and the NF-κB pathway. These results provide the rationale for further FC2 modifications and the design of novel IAP-targeting candidates supporting known therapies.
Keywords: MDA-MB-231; NF-κB (nuclear factor kappa light chain enhancer of activated B cells); baculoviral IAP repeat (BIR); benzodiazepinone; inhibitor of apoptosis protein (IAP); virtual docking.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Structure-based identification of a new IAP-targeting compound that induces cancer cell death inducing NF-κB pathway.Comput Struct Biotechnol J. 2021 Nov 26;19:6366-6374. doi: 10.1016/j.csbj.2021.11.034. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34938412 Free PMC article.
-
A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells.Oncogene. 2005 Nov 10;24(49):7381-8. doi: 10.1038/sj.onc.1208888. Oncogene. 2005. PMID: 16044155
-
Characterization of Potent SMAC Mimetics that Sensitize Cancer Cells to TNF Family-Induced Apoptosis.PLoS One. 2016 Sep 12;11(9):e0161952. doi: 10.1371/journal.pone.0161952. eCollection 2016. PLoS One. 2016. PMID: 27617834 Free PMC article.
-
Small-molecule SMAC mimetics as new cancer therapeutics.Pharmacol Ther. 2014 Oct;144(1):82-95. doi: 10.1016/j.pharmthera.2014.05.007. Epub 2014 May 16. Pharmacol Ther. 2014. PMID: 24841289 Free PMC article. Review.
-
Smac mimetics and TNFalpha: a dangerous liaison?Cell. 2007 Nov 16;131(4):655-8. doi: 10.1016/j.cell.2007.10.042. Cell. 2007. PMID: 18022360 Free PMC article. Review.
References
-
- Greenblatt D.J., Shader R.I., Abernethy D.R. Drug Therapy. Current Status of Benzodiazepines. N. Engl. J. Med. 1983;309:354–358. - PubMed
-
- Henderson E.A., Alber D.G., Baxter R.C., Bithell S.K., Budworth J., Carter M.C., Chubb A., Cockerill G.S., Dowdell V.C.L., Fraser I.J., et al. 1,4-Benzodiazepines as Inhibitors of Respiratory Syncytial Virus. The Identification of a Clinical Candidate. J. Med. Chem. 2007;50:1685–1692. doi: 10.1021/jm060747l. - DOI - PubMed
-
- Micale N., Colleoni S., Postorino G., Pellicanò A., Zappalà M., Lazzaro J., Diana V., Cagnotto A., Mennini T., Grasso S. Structure-Activity Study of 2,3-Benzodiazepin-4-Ones Noncompetitive AMPAR Antagonists: Identification of the 1-(4-Amino-3-Methylphenyl)-3,5-Dihydro-7,8-Ethylenedioxy-4H-2,3-Benzodiazepin-4-One as Neuroprotective Agent. Bioorg. Med. Chem. 2008;16:2200–2211. doi: 10.1016/j.bmc.2007.11.080. - DOI - PubMed
-
- Leonard K., Marugan J.J., Raboisson P., Calvo R., Gushue J.M., Koblish H.K., Lattanze J., Zhao S., Cummings M.D., Player M.R., et al. Novel 1,4-Benzodiazepine-2,5-Diones as Hdm2 Antagonists with Improved Cellular Activity. Bioorg. Med. Chem. Lett. 2006;16:3463–3468. doi: 10.1016/j.bmcl.2006.04.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

