CRAC and SK Channels: Their Molecular Mechanisms Associated with Cancer Cell Development
- PMID: 36612099
- PMCID: PMC9817886
- DOI: 10.3390/cancers15010101
CRAC and SK Channels: Their Molecular Mechanisms Associated with Cancer Cell Development
Abstract
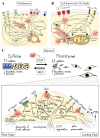
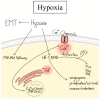
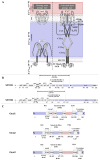
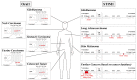
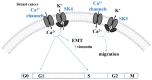
Cancer represents a major health burden worldwide. Several molecular targets have been discovered alongside treatments with positive clinical outcomes. However, the reoccurrence of cancer due to therapy resistance remains the primary cause of mortality. Endeavors in pinpointing new markers as molecular targets in cancer therapy are highly desired. The significance of the co-regulation of Ca2+-permeating and Ca2+-regulated ion channels in cancer cell development, proliferation, and migration make them promising molecular targets in cancer therapy. In particular, the co-regulation of the Orai1 and SK3 channels has been well-studied in breast and colon cancer cells, where it finally leads to an invasion-metastasis cascade. Nevertheless, many questions remain unanswered, such as which key molecular components determine and regulate their interplay. To provide a solid foundation for a better understanding of this ion channel co-regulation in cancer, we first shed light on the physiological role of Ca2+ and how this ion is linked to carcinogenesis. Then, we highlight the structure/function relationship of Orai1 and SK3, both individually and in concert, their role in the development of different types of cancer, and aspects that are not yet known in this context.
Keywords: CRAC channel; SK3 channel; cancer; cancer hallmarks; cancer signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
How to create accessible research summaries for the developmental language disorder community.Int J Lang Commun Disord. 2025 Jan-Feb;60(1):e13142. doi: 10.1111/1460-6984.13142. Int J Lang Commun Disord. 2025. PMID: 39625399
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
S-acylation of Ca2+ transport proteins in cancer.Chronic Dis Transl Med. 2024 Aug 14;10(4):263-280. doi: 10.1002/cdt3.146. eCollection 2024 Dec. Chronic Dis Transl Med. 2024. PMID: 39429488 Free PMC article. Review.
-
Synthetic Biology Meets Ca2+ Release-Activated Ca2+ Channel-Dependent Immunomodulation.Cells. 2024 Mar 7;13(6):468. doi: 10.3390/cells13060468. Cells. 2024. PMID: 38534312 Free PMC article. Review.
-
Cannabigerolic Acid (CBGA) Inhibits the TRPM7 Ion Channel Through its Kinase Domain.Function (Oxf). 2023 Dec 7;5(1):zqad069. doi: 10.1093/function/zqad069. eCollection 2024. Function (Oxf). 2023. PMID: 38162115 Free PMC article.
-
The Bis(1,2,3,4-tetrahydroisoquinoline) Alkaloids Cepharanthine and Berbamine Are Ligands of SK Channels.ACS Med Chem Lett. 2024 Jan 9;15(2):215-220. doi: 10.1021/acsmedchemlett.3c00452. eCollection 2024 Feb 8. ACS Med Chem Lett. 2024. PMID: 38352826 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

