Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies
- PMID: 36612019
- PMCID: PMC9818030
- DOI: 10.3390/cancers15010023
Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies
Abstract
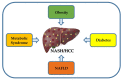
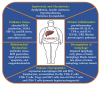
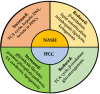
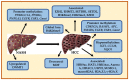
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is the sixth most frequent cancer in the world, being the third cause of cancer-related deaths. Nonalcoholic steatohepatitis (NASH) is characterized by fatty infiltration, oxidative stress and necroinflammation of the liver, with or without fibrosis, which can progress to advanced liver fibrosis, cirrhosis and HCC. Obesity, metabolic syndrome, insulin resistance, and diabetes exacerbates the course of NASH, which elevate the risk of HCC. The growing prevalence of obesity are related with increasing incidence of NASH, which may play a growing role in HCC epidemiology worldwide. In addition, HCC initiation and progression is driven by reprogramming of metabolism, which indicates growing appreciation of metabolism in the pathogenesis of this disease. Although no specific preventive pharmacological treatments have recommended for NASH, dietary restriction and exercise are recommended. This review focuses on the molecular connections between HCC and NASH, including genetic and risk factors, highlighting the metabolic reprogramming and aberrant epigenetic alterations in the development of HCC in NASH. Current therapeutic aspects of NASH/HCC are also reviewed.
Keywords: current therapeutic strategies; epidemiology; epigenetic alterations; genetic and risk factors; hepatocellular carcinoma; metabolic reprogramming; nonalcoholic steatohepatitis; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma.Int J Mol Sci. 2020 Feb 23;21(4):1525. doi: 10.3390/ijms21041525. Int J Mol Sci. 2020. PMID: 32102237 Free PMC article. Review.
-
Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection.Hepatology. 2010 May;51(5):1820-32. doi: 10.1002/hep.23594. Hepatology. 2010. PMID: 20432259 Review.
-
State of CD8+ T cells in progression from nonalcoholic steatohepatitis to hepatocellular carcinoma: From pathogenesis to immunotherapy.Biomed Pharmacother. 2023 Sep;165:115131. doi: 10.1016/j.biopha.2023.115131. Epub 2023 Jul 8. Biomed Pharmacother. 2023. PMID: 37429231 Review.
-
Molecular Pathogenesis of Nonalcoholic Steatohepatitis- (NASH-) Related Hepatocellular Carcinoma.Can J Gastroenterol Hepatol. 2018 Aug 29;2018:8543763. doi: 10.1155/2018/8543763. eCollection 2018. Can J Gastroenterol Hepatol. 2018. PMID: 30228976 Free PMC article. Review.
-
Advances in hepatocellular carcinoma: Nonalcoholic steatohepatitis-related hepatocellular carcinoma.World J Hepatol. 2015 Aug 28;7(18):2155-61. doi: 10.4254/wjh.v7.i18.2155. World J Hepatol. 2015. PMID: 26328027 Free PMC article. Review.
Cited by
-
The Role of RNA Splicing in Liver Function and Disease: A Focus on Metabolic Dysfunction-Associated Steatotic Liver Disease.Genes (Basel). 2024 Sep 8;15(9):1181. doi: 10.3390/genes15091181. Genes (Basel). 2024. PMID: 39336772 Free PMC article. Review.
-
Associations of Relative Fat Mass, a Novel Adiposity Indicator, with Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: Data from SPECT-China.Diabetes Metab Syndr Obes. 2023 Aug 8;16:2377-2387. doi: 10.2147/DMSO.S423272. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37577042 Free PMC article.
-
High-density lipoprotein cholesterol trajectory and new-onset metabolic dysfunction-associated fatty liver disease incidence: a longitudinal study.Diabetol Metab Syndr. 2024 Sep 11;16(1):223. doi: 10.1186/s13098-024-01457-y. Diabetol Metab Syndr. 2024. PMID: 39261925 Free PMC article.
-
Circ_0084615 promotes epithelial-mesenchymal transition-mediated tumor progression in hepatocellular carcinoma.Ann Gastroenterol Surg. 2024 Jun 3;8(6):1107-1117. doi: 10.1002/ags3.12828. eCollection 2024 Nov. Ann Gastroenterol Surg. 2024. PMID: 39502735 Free PMC article.
-
The NRF-2/HO-1 Signaling Pathway: A Promising Therapeutic Target for Metabolic Dysfunction-Associated Steatotic Liver Disease.J Inflamm Res. 2024 Nov 3;17:8061-8083. doi: 10.2147/JIR.S490418. eCollection 2024. J Inflamm Res. 2024. PMID: 39512865 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

