The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders
- PMID: 36611848
- PMCID: PMC9818777
- DOI: 10.3390/cells12010054
The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders
Abstract
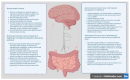
Mounting evidence shows that the complex gut microbial ecosystem in the human gastrointestinal (GI) tract regulates the physiology of the central nervous system (CNS) via microbiota and the gut-brain (MGB) axis. The GI microbial ecosystem communicates with the brain through the neuroendocrine, immune, and autonomic nervous systems. Recent studies have bolstered the involvement of dysfunctional MGB axis signaling in the pathophysiology of several neurodegenerative, neurodevelopmental, and neuropsychiatric disorders (NPDs). Several investigations on the dynamic microbial system and genetic-environmental interactions with the gut microbiota (GM) have shown that changes in the composition, diversity and/or functions of gut microbes (termed "gut dysbiosis" (GD)) affect neuropsychiatric health by inducing alterations in the signaling pathways of the MGB axis. Interestingly, both preclinical and clinical evidence shows a positive correlation between GD and the pathogenesis and progression of NPDs. Long-term GD leads to overstimulation of hypothalamic-pituitary-adrenal (HPA) axis and the neuroimmune system, along with altered neurotransmitter levels, resulting in dysfunctional signal transduction, inflammation, increased oxidative stress (OS), mitochondrial dysfunction, and neuronal death. Further studies on the MGB axis have highlighted the significance of GM in the development of brain regions specific to stress-related behaviors, including depression and anxiety, and the immune system in the early life. GD-mediated deregulation of the MGB axis imbalances host homeostasis significantly by disrupting the integrity of the intestinal and blood-brain barrier (BBB), mucus secretion, and gut immune and brain immune functions. This review collates evidence on the potential interaction between GD and NPDs from preclinical and clinical data. Additionally, we summarize the use of non-therapeutic modulators such as pro-, pre-, syn- and post-biotics, and specific diets or fecal microbiota transplantation (FMT), which are promising targets for the management of NPDs.
Keywords: gut dysbiosis; gut microbiota; inflammation; microbiota gut–brain axis; neuropsychiatric disorders; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation.Clin Ther. 2015 May 1;37(5):984-95. doi: 10.1016/j.clinthera.2015.04.002. Clin Ther. 2015. PMID: 26046241 Free PMC article. Review.
-
Gut-brain axis: A matter of concern in neuropsychiatric disorders…!Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jan 10;104:110051. doi: 10.1016/j.pnpbp.2020.110051. Epub 2020 Aug 3. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32758517 Review.
-
Neuropsychiatric Ramifications of COVID-19: Short-Chain Fatty Acid Deficiency and Disturbance of Microbiota-Gut-Brain Axis Signaling.Biomed Res Int. 2021 Oct 5;2021:7880448. doi: 10.1155/2021/7880448. eCollection 2021. Biomed Res Int. 2021. PMID: 34651049 Free PMC article. Review.
-
Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jul 13;109:110258. doi: 10.1016/j.pnpbp.2021.110258. Epub 2021 Jan 23. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33497754 Review.
-
Microbiota-gut brain axis involvement in neuropsychiatric disorders.Expert Rev Neurother. 2019 Oct;19(10):1037-1050. doi: 10.1080/14737175.2019.1638763. Epub 2019 Jul 11. Expert Rev Neurother. 2019. PMID: 31260640 Review.
Cited by
-
Gut-Brain Axis and Psychopathology: Exploring the Impact of Diet with a Focus on the Low-FODMAP Approach.Nutrients. 2024 Oct 17;16(20):3515. doi: 10.3390/nu16203515. Nutrients. 2024. PMID: 39458509 Free PMC article. Review.
-
Probiotics by Modulating Gut-Brain Axis Together With Brivaracetam Mitigate Seizure Progression, Behavioral Incongruities, and Prevented Neurodegeneration in Pentylenetetrazole-Kindled Mice.CNS Neurosci Ther. 2024 Nov;30(11):e70078. doi: 10.1111/cns.70078. CNS Neurosci Ther. 2024. PMID: 39470120 Free PMC article.
-
Brain-Gut and Microbiota-Gut-Brain Communication in Type-2 Diabetes Linked Alzheimer's Disease.Nutrients. 2024 Aug 3;16(15):2558. doi: 10.3390/nu16152558. Nutrients. 2024. PMID: 39125436 Free PMC article. Review.
-
Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression.Biomedicines. 2023 Sep 22;11(10):2600. doi: 10.3390/biomedicines11102600. Biomedicines. 2023. PMID: 37892974 Free PMC article.
-
Epidemiology of small intestinal bacterial overgrowth.World J Gastroenterol. 2023 Jun 14;29(22):3400-3421. doi: 10.3748/wjg.v29.i22.3400. World J Gastroenterol. 2023. PMID: 37389240 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

