Neutrophil Extracellular Traps Delay Diabetic Wound Healing by Inducing Endothelial-to-Mesenchymal Transition via the Hippo pathway
- PMID: 36594092
- PMCID: PMC9760440
- DOI: 10.7150/ijbs.78046
Neutrophil Extracellular Traps Delay Diabetic Wound Healing by Inducing Endothelial-to-Mesenchymal Transition via the Hippo pathway
Abstract
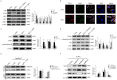
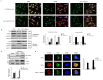
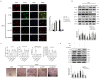
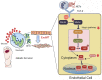
Diabetic foot ulcers (DFUs) are among the most frequent complications of diabetes with significant morbidity and mortality. Diabetes can trigger neutrophils to undergo histone citrullination by protein arginine deiminase 4 (encoded by Padi4 in mice) and release neutrophil extracellular traps (NETs). The specific mechanism of NETs-mediated wound healing impairment in diabetes remains unknown. In this study, we show neutrophils are more susceptible to NETosis in diabetic wound environments. Via in vitro experiments and in vivo models of wound healing using wide-type and Padi4 -/- mice, we demonstrate NETs can induce the activation of PAK2 via the membrane receptor TLR-9. Then PAK2 phosphorylates the intracellular protein Merlin/NF2 to inhibit the Hippo-YAP pathway. YAP binds to transcription factor SMAD2 and translocates from the cytoplasm into the nucleus to promote endothelial-to-mesenchymal transition (EndMT), which ultimately impedes angiogenesis and delays wound healing. Suppression of the Merlin/YAP/SMAD2 pathway can attenuate NET-induced EndMT. Inhibition of NETosis accelerates wound healing by reducing EndMT and promoting angiogenesis. Cumulatively, these data suggest NETosis delays diabetic wound healing by inducing EndMT via the Hippo-YAP pathway. Increased understanding of the molecular mechanism that regulates NETosis and EndMT will be of considerable value for providing cellular targets amenable to therapeutic intervention for DFUs.
Keywords: Hippo pathway; diabetic foot ulcer; endothelial-to-mesenchymal transition; neutrophil extracellular traps; wound healing.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
NETosis Delays Diabetic Wound Healing in Mice and Humans.Diabetes. 2016 Apr;65(4):1061-71. doi: 10.2337/db15-0863. Epub 2016 Jan 6. Diabetes. 2016. PMID: 26740598
-
Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis.Mol Cell Endocrinol. 2019 Jan 15;480:74-82. doi: 10.1016/j.mce.2018.10.013. Epub 2018 Oct 16. Mol Cell Endocrinol. 2019. PMID: 30339820
-
Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway.Transl Res. 2023 Apr;254:115-127. doi: 10.1016/j.trsl.2022.10.008. Epub 2022 Nov 3. Transl Res. 2023. PMID: 36336332
-
Attack of the NETs! NETosis primes IL-1β-mediated inflammation in diabetic foot ulcers.Clin Sci (Lond). 2020 Jun 26;134(12):1399-1401. doi: 10.1042/CS20200240. Clin Sci (Lond). 2020. PMID: 32556177 Review.
-
NETosis contributes to the pathogenesis of diabetes and its complications.J Mol Endocrinol. 2020 Nov;65(4):R65-R76. doi: 10.1530/JME-20-0128. J Mol Endocrinol. 2020. PMID: 33048064 Review.
Cited by
-
Neutrophils extracellular traps and ferroptosis in diabetic wounds.Int Wound J. 2023 Nov;20(9):3840-3854. doi: 10.1111/iwj.14231. Epub 2023 May 17. Int Wound J. 2023. PMID: 37199077 Free PMC article. Review.
-
Neutrophil extracellular traps in homeostasis and disease.Signal Transduct Target Ther. 2024 Sep 20;9(1):235. doi: 10.1038/s41392-024-01933-x. Signal Transduct Target Ther. 2024. PMID: 39300084 Free PMC article. Review.
-
LINC01117 inhibits invasion and migration of lung adenocarcinoma through influencing EMT process.PLoS One. 2023 Jun 29;18(6):e0287926. doi: 10.1371/journal.pone.0287926. eCollection 2023. PLoS One. 2023. PMID: 37384755 Free PMC article.
-
Endothelial dysfunction in vascular complications of diabetes: a comprehensive review of mechanisms and implications.Front Endocrinol (Lausanne). 2024 Apr 5;15:1359255. doi: 10.3389/fendo.2024.1359255. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38645427 Free PMC article. Review.
-
Exosome-based cell therapy for diabetic foot ulcers: Present and prospect.Heliyon. 2024 Oct 11;10(20):e39251. doi: 10.1016/j.heliyon.2024.e39251. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498056 Free PMC article. Review.
References
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet (London, England) 2005;366(9498):1736–43. - PubMed
-
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15(11):599–607. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS. et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

