Nanoparticles augment the therapeutic window of RT and immunotherapy for treating cancers: pivotal role of autophagy
- PMID: 36593951
- PMCID: PMC9800737
- DOI: 10.7150/thno.77233
Nanoparticles augment the therapeutic window of RT and immunotherapy for treating cancers: pivotal role of autophagy
Abstract
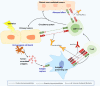
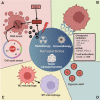
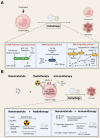
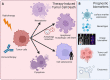
Immunotherapies are now emerging as an efficient anticancer therapeutic strategy. Cancer immunotherapy utilizes the host's immune system to fight against cancer cells and has gained increasing interest due to its durable efficacy and low toxicity compared to traditional antitumor treatments, such as chemotherapy and radiotherapy (RT). Although the combination of RT and immunotherapy has drawn extensive attention in the clinical setting, the overall response rates are still low. Therefore, strategies for further improvement are urgently needed. Nanotechnology has been used in cancer immunotherapy and RT to target not only cancer cells but also the tumor microenvironment (TME), thereby helping to generate a long-term immune response. Nanomaterials can be an effective delivery system and a strong autophagy inducer, with the ability to elevate autophagy to very high levels. Interestingly, autophagy could play a critical role in optimal immune function, mediating cell-extrinsic homeostatic effects through the regulation of danger signaling in neoplastic cells under immunogenic chemotherapy and/or RT. In this review, we summarize the preclinical and clinical development of the combination of immunotherapy and RT in cancer therapy and highlight the latest progress in nanotechnology for augmenting the anticancer effects of immunotherapy and RT. The underlying mechanisms of nanomaterial-triggered autophagy in tumor cells and the TME are discussed in depth. Finally, we suggest the implications of these three strategies combined together to achieve the goal of maximizing the therapeutic advantages of cancer therapy and show recent advances in biomarkers for tumor response in the evaluation of those therapies.
Keywords: autophagy; immunotherapy; nanomaterials; radiotherapy; tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Nanoscale Metal-Organic Frameworks for Cancer Immunotherapy.Acc Chem Res. 2020 Sep 15;53(9):1739-1748. doi: 10.1021/acs.accounts.0c00313. Epub 2020 Aug 18. Acc Chem Res. 2020. PMID: 32808760 Free PMC article.
-
Exploiting autophagy-regulative nanomaterials for activation of dendritic cells enables reinforced cancer immunotherapy.Biomaterials. 2022 Mar;282:121434. doi: 10.1016/j.biomaterials.2022.121434. Epub 2022 Feb 18. Biomaterials. 2022. PMID: 35202930 Review.
-
Emerging role of autophagy in anti-tumor immunity: Implications for the modulation of immunotherapy resistance.Drug Resist Updat. 2021 May;56:100752. doi: 10.1016/j.drup.2021.100752. Epub 2021 Feb 16. Drug Resist Updat. 2021. PMID: 33765484 Review.
-
Nanomaterial-Based Modulation of Tumor Microenvironments for Enhancing Chemo/Immunotherapy.AAPS J. 2019 May 17;21(4):64. doi: 10.1208/s12248-019-0333-y. AAPS J. 2019. PMID: 31102154 Review.
-
Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy.Front Immunol. 2022 Oct 10;13:1018903. doi: 10.3389/fimmu.2022.1018903. eCollection 2022. Front Immunol. 2022. PMID: 36300110 Free PMC article. Review.
Cited by
-
GdVO4:Eu3+ and LaVO4:Eu3+ Nanoparticles Exacerbate Oxidative Stress in L929 Cells: Potential Implications for Cancer Therapy.Int J Mol Sci. 2024 Oct 30;25(21):11687. doi: 10.3390/ijms252111687. Int J Mol Sci. 2024. PMID: 39519237 Free PMC article.
-
Mechanisms and therapeutic targets of ferroptosis: Implications for nanomedicine design.J Pharm Anal. 2024 Jul;14(7):100960. doi: 10.1016/j.jpha.2024.03.001. Epub 2024 Mar 8. J Pharm Anal. 2024. PMID: 39135963 Free PMC article. Review.
-
Modulation of the immunity and inflammation by autophagy.MedComm (2020). 2023 Jul 2;4(4):e311. doi: 10.1002/mco2.311. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37405276 Free PMC article. Review.
-
Systematic Studies on Anti-Cancer Evaluation of Stilbene and Dibenzo[b,f]oxepine Derivatives.Molecules. 2023 Apr 18;28(8):3558. doi: 10.3390/molecules28083558. Molecules. 2023. PMID: 37110792 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

