In vitro elastic cartilage reconstruction using human auricular perichondrial chondroprogenitor cell-derived micro 3D spheroids
- PMID: 36582939
- PMCID: PMC9793062
- DOI: 10.1177/20417314221143484
In vitro elastic cartilage reconstruction using human auricular perichondrial chondroprogenitor cell-derived micro 3D spheroids
Abstract
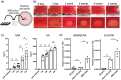
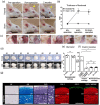
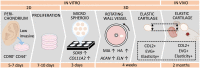
Morphologically stable scaffold-free elastic cartilage tissue is crucial for treating external ear abnormalities. However, establishing adequate mechanical strength is challenging, owing to the difficulty of achieving chondrogenic differentiation in vitro; thus, cartilage reconstruction is a complex task. Auricular perichondrial chondroprogenitor cells exhibit high proliferation potential and can be obtained with minimal invasion. Therefore, these cells are an ideal resource for elastic cartilage reconstruction. In this study, we aimed to develop a novel in vitro scaffold-free method for elastic cartilage reconstruction, using human auricular perichondrial chondroprogenitor cells. Inducing chondrogenesis by using microscopic spheroids similar to auricular hillocks significantly increased the chondrogenic potential. The size and elasticity of the tissue were maintained after craniofacial transplantation in immunodeficient mice, suggesting that the reconstructed tissue was morphologically stable. Our novel tissue reconstruction method may facilitate the development of future treatments for external ear abnormalities.
Keywords: Elastic cartilage; auricular perichondrial chondroprogenitor cells; micro three-dimensional culture; microtia; rotating culture.
© The Author(s) 2022.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
The application and progress of stem cells in auricular cartilage regeneration: a systematic review.Front Cell Dev Biol. 2023 Jul 26;11:1204050. doi: 10.3389/fcell.2023.1204050. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37564374 Free PMC article. Review.
-
Engineering of scaffold-free tri-layered auricular tissues for external ear reconstruction.Laryngoscope. 2019 Aug;129(8):E272-E283. doi: 10.1002/lary.27823. Epub 2019 Jan 30. Laryngoscope. 2019. PMID: 30698822
-
The application and progress of tissue engineering and biomaterial scaffolds for total auricular reconstruction in microtia.Front Bioeng Biotechnol. 2023 Sep 22;11:1089031. doi: 10.3389/fbioe.2023.1089031. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37811379 Free PMC article. Review.
-
Development of a Method for Scaffold-Free Elastic Cartilage Creation.Int J Mol Sci. 2020 Nov 11;21(22):8496. doi: 10.3390/ijms21228496. Int J Mol Sci. 2020. PMID: 33187369 Free PMC article.
-
Characterization of pediatric microtia cartilage: a reservoir of chondrocytes for auricular reconstruction using tissue engineering strategies.Cell Tissue Bank. 2016 Sep;17(3):481-9. doi: 10.1007/s10561-016-9574-5. Epub 2016 Aug 26. Cell Tissue Bank. 2016. PMID: 27566509
Cited by
-
Suitability of Ex Vivo-Expanded Microtic Perichondrocytes for Auricular Reconstruction.Cells. 2024 Jan 12;13(2):141. doi: 10.3390/cells13020141. Cells. 2024. PMID: 38247833 Free PMC article.
-
The application and progress of stem cells in auricular cartilage regeneration: a systematic review.Front Cell Dev Biol. 2023 Jul 26;11:1204050. doi: 10.3389/fcell.2023.1204050. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37564374 Free PMC article. Review.
-
Craniofacial chondrogenesis in organoids from human stem cell-derived neural crest cells.iScience. 2024 Mar 28;27(4):109585. doi: 10.1016/j.isci.2024.109585. eCollection 2024 Apr 19. iScience. 2024. PMID: 38623327 Free PMC article.
References
-
- Griffin MF, O’Toole G, Sabbagh W, et al.. Comparison of the compressive mechanical properties of auricular and costal cartilage from patients with microtia. J Biomech 2020; 103: 109688. - PubMed
LinkOut - more resources
Full Text Sources

