Glucose metabolism and tumour microenvironment in pancreatic cancer: A key link in cancer progression
- PMID: 36578477
- PMCID: PMC9792100
- DOI: 10.3389/fimmu.2022.1038650
Glucose metabolism and tumour microenvironment in pancreatic cancer: A key link in cancer progression
Abstract
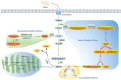
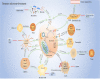
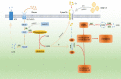
Early and accurate diagnosis and treatment of pancreatic cancer (PC) remain challenging endeavors globally. Late diagnosis lag, high invasiveness, chemical resistance, and poor prognosis are unresolved issues of PC. The concept of metabolic reprogramming is a hallmark of cancer cells. Increasing evidence shows that PC cells alter metabolic processes such as glucose, amino acids, and lipids metabolism and require continuous nutrition for survival, proliferation, and invasion. Glucose metabolism, in particular, regulates the tumour microenvironment (TME). Furthermore, the link between glucose metabolism and TME also plays an important role in the targeted therapy, chemoresistance, radiotherapy ineffectiveness, and immunosuppression of PC. Altered metabolism with the TME has emerged as a key mechanism regulating PC progression. This review shed light on the relationship between TME, glucose metabolism, and various aspects of PC. The findings of this study provide a new direction in the development of PC therapy targeting the metabolism of cancer cells.
Keywords: glucose metabolism; pancreatic cancer; prognosis; treatment; tumour microenvironment.
Copyright © 2022 Dong, Li, Li, Wang, Chen, Shi, He, Chen and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cellular metabolism in pancreatic cancer as a tool for prognosis and treatment (Review).Int J Oncol. 2022 Aug;61(2):93. doi: 10.3892/ijo.2022.5383. Epub 2022 Jun 22. Int J Oncol. 2022. PMID: 35730611 Free PMC article. Review.
-
Metabolic reprogramming due to hypoxia in pancreatic cancer: Implications for tumor formation, immunity, and more.Biomed Pharmacother. 2021 Sep;141:111798. doi: 10.1016/j.biopha.2021.111798. Epub 2021 Jun 10. Biomed Pharmacother. 2021. PMID: 34120068 Review.
-
miR-590-5p/Tiam1-mediated glucose metabolism promotes malignant evolution of pancreatic cancer by regulating SLC2A3 stability.Cancer Cell Int. 2023 Nov 28;23(1):301. doi: 10.1186/s12935-023-03159-3. Cancer Cell Int. 2023. PMID: 38017477 Free PMC article.
-
Cancer-associated fibroblasts: A key target to snatch victory from defeat in therapy resistance associated with the pancreatic cancer stroma.Cancer Lett. 2023 Jul 28;567:216279. doi: 10.1016/j.canlet.2023.216279. Epub 2023 Jun 17. Cancer Lett. 2023. PMID: 37336287 Review.
-
Hurdle or thruster: Glucose metabolism of T cells in anti-tumour immunity.Biochim Biophys Acta Rev Cancer. 2024 Jan;1879(1):189022. doi: 10.1016/j.bbcan.2023.189022. Epub 2023 Nov 20. Biochim Biophys Acta Rev Cancer. 2024. PMID: 37993001 Review.
Cited by
-
Extranodal lymphoma: pathogenesis, diagnosis and treatment.Mol Biomed. 2023 Sep 18;4(1):29. doi: 10.1186/s43556-023-00141-3. Mol Biomed. 2023. PMID: 37718386 Free PMC article. Review.
-
GSH-responsive degradable nanodrug for glucose metabolism intervention and induction of ferroptosis to enhance magnetothermal anti-tumor therapy.J Nanobiotechnology. 2024 Apr 3;22(1):147. doi: 10.1186/s12951-024-02425-4. J Nanobiotechnology. 2024. PMID: 38570829 Free PMC article.
References
-
- Bednar F, Pasca di Magliano M. Chemotherapy and tumor evolution shape pancreatic cancer recurrence after resection. Cancer Discovery (2020) 10(6):762–4. doi: 10.1158/2159-8290.CD-20-0359 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

