Tanshinone IIA Improves Acute Gouty Arthritis in Rats through Regulating Neutrophil Activation and the NLRP3 Inflammasome
- PMID: 36578443
- PMCID: PMC9792249
- DOI: 10.1155/2022/5851412
Tanshinone IIA Improves Acute Gouty Arthritis in Rats through Regulating Neutrophil Activation and the NLRP3 Inflammasome
Abstract
Objectives: To investigate the prevention and treatment effect of tanshinone IIA (TIIA) on acute gouty arthritis (AGA) and its mechanism.
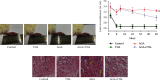
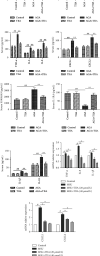
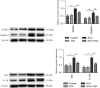
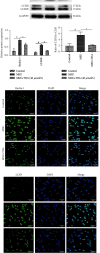
Methods: The anti-AGA effect of TIIA was observed in vivo and in vitro. Neutrophils were isolated from the abdominal cavity of mice, and the anti-AGA effect of TIIA was investigated in a rat model of MSU-induced AGA. The pathological changes of the ankle joint tissues were assessed by H&E. Cytokine and chemokine expression were determined by ELISA and RT-qPCR. The NLRP3 inflammasome pathway protein levels in the ankle joint tissues were evaluated via western blotting. Neutrophil migration was evaluated in air pouch and transwell assays. Immunohistochemistry and immunofluorescence analysis evaluate the release of myeloperoxidase (MPO), neutrophil elastase (NE), and citrullination of histone H3 (CitH3). Beclin-1 and LC3B expressions were determined using western blotting and immunofluorescence. Key Findings. Treatment with TIIA alleviated synovial hyperplasia and neutrophil infiltration, regulated cytokine and chemokine expressions, and inhibited NLRP3 activation in AGA rats, neutrophil migration, MPO, NE, and CitH3 expression, and LC3B and Beclin-1 protein expression.
Conclusions: These results demonstrate that TIIA can effectively enhance AGA by focusing on the neutrophils and NLRP3 inflammasome, demonstrating that TIIA may act as a potential helpful agent for AGA.
Copyright © 2022 Lianjie Xu et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Triptolide alleviates acute gouty arthritis caused by monosodium urate crystals by modulating macrophage polarization and neutrophil activity.Immunol Lett. 2024 Oct;269:106907. doi: 10.1016/j.imlet.2024.106907. Epub 2024 Aug 8. Immunol Lett. 2024. PMID: 39122094
-
Curcumin ameliorates monosodium urate-induced gouty arthritis through Nod-like receptor 3 inflammasome mediation via inhibiting nuclear factor-kappa B signaling.J Cell Biochem. 2019 Apr;120(4):6718-6728. doi: 10.1002/jcb.27969. Epub 2018 Dec 28. J Cell Biochem. 2019. PMID: 30592318
-
Modified Simiaowan prevents and treats gouty arthritis via the Nrf2/NLRP3 inflammasome signaling pathway.J Ethnopharmacol. 2024 Jan 10;318(Pt A):116906. doi: 10.1016/j.jep.2023.116906. Epub 2023 Jul 11. J Ethnopharmacol. 2024. PMID: 37442492
-
Autophagy induced by PP121 alleviates MSU crystal-induced acute gouty arthritis via inhibition of the NLRP3 inflammasome.Int Immunopharmacol. 2023 Oct;123:110756. doi: 10.1016/j.intimp.2023.110756. Epub 2023 Aug 11. Int Immunopharmacol. 2023. PMID: 37573689
-
Traditional herbal medicine: Therapeutic potential in acute gouty arthritis.J Ethnopharmacol. 2024 Aug 10;330:118182. doi: 10.1016/j.jep.2024.118182. Epub 2024 Apr 15. J Ethnopharmacol. 2024. PMID: 38621464 Review.
Cited by
-
TCM and related active compounds in the treatment of gout: the regulation of signaling pathway and urate transporter.Front Pharmacol. 2023 Nov 29;14:1275974. doi: 10.3389/fphar.2023.1275974. eCollection 2023. Front Pharmacol. 2023. PMID: 38094893 Free PMC article. Review.
-
Possible mechanisms of treatment for spinal cord injury repair with tanshinone IIA.Front Mol Neurosci. 2023 Sep 21;16:1277755. doi: 10.3389/fnmol.2023.1277755. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37808475 Free PMC article.
-
Exploring the inhibition mechanism of interleukin-1-beta in gouty arthritis by polygonum cuspidatum using network pharmacology and molecular docking: A review.Medicine (Baltimore). 2023 Jul 21;102(29):e34396. doi: 10.1097/MD.0000000000034396. Medicine (Baltimore). 2023. PMID: 37478249 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

