Insights into the tumor microenvironment of B cell lymphoma
- PMID: 36578079
- PMCID: PMC9798587
- DOI: 10.1186/s13046-022-02579-9
Insights into the tumor microenvironment of B cell lymphoma
Abstract
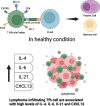
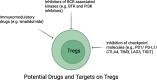
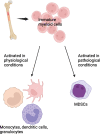
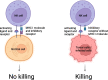
The standard therapies in lymphoma have predominantly focused on targeting tumor cells with less of a focus on the tumor microenvironment (TME), which plays a critical role in favoring tumor growth and survival. Such an approach may result in increasingly refractory disease with progressively reduced responses to subsequent treatments. To overcome this hurdle, targeting the TME has emerged as a new therapeutic strategy. The TME consists of T and B lymphocytes, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts (CAFs), and other components. Understanding the TME can lead to a comprehensive approach to managing lymphoma, resulting in therapeutic strategies that target not only cancer cells, but also the supportive environment and thereby ultimately improve survival of lymphoma patients. Here, we review the normal function of different components of the TME, the impact of their aberrant behavior in B cell lymphoma and the current TME-direct therapeutic avenues.
Keywords: B-cell lymphoma; Cancer-associated fibroblasts; Myeloid-derived suppressor cells; T cells; T follicular helper cells; T regulatory cells; Tumor microenvironment; Tumor-associated macrophages.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilized in the production of this manuscript.
Figures


Similar articles
-
Unraveling the Immune Microenvironment in Diffuse Large B-Cell Lymphoma: Prognostic and Potential Therapeutic Implications.Curr Issues Mol Biol. 2024 Jul 5;46(7):7048-7064. doi: 10.3390/cimb46070420. Curr Issues Mol Biol. 2024. PMID: 39057061 Free PMC article. Review.
-
Diamonds in the Rough: Harnessing Tumor-Associated Myeloid Cells for Cancer Therapy.Front Immunol. 2018 Oct 8;9:2250. doi: 10.3389/fimmu.2018.02250. eCollection 2018. Front Immunol. 2018. PMID: 30349530 Free PMC article. Review.
-
Forces at play: exploring factors affecting the cancer metastasis.Front Immunol. 2024 Feb 1;15:1274474. doi: 10.3389/fimmu.2024.1274474. eCollection 2024. Front Immunol. 2024. PMID: 38361941 Free PMC article. Review.
-
The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment.Bioact Mater. 2020 Dec 26;6(7):1973-1987. doi: 10.1016/j.bioactmat.2020.12.010. eCollection 2021 Jul. Bioact Mater. 2020. PMID: 33426371 Free PMC article. Review.
-
Modulation of the tumor microenvironment (TME) by melatonin.Eur J Pharmacol. 2021 Sep 15;907:174365. doi: 10.1016/j.ejphar.2021.174365. Epub 2021 Jul 21. Eur J Pharmacol. 2021. PMID: 34302814 Review.
Cited by
-
Unraveling the Immune Microenvironment in Diffuse Large B-Cell Lymphoma: Prognostic and Potential Therapeutic Implications.Curr Issues Mol Biol. 2024 Jul 5;46(7):7048-7064. doi: 10.3390/cimb46070420. Curr Issues Mol Biol. 2024. PMID: 39057061 Free PMC article. Review.
-
Potential Pathogenic Impact of Cow's Milk Consumption and Bovine Milk-Derived Exosomal MicroRNAs in Diffuse Large B-Cell Lymphoma.Int J Mol Sci. 2023 Mar 23;24(7):6102. doi: 10.3390/ijms24076102. Int J Mol Sci. 2023. PMID: 37047075 Free PMC article. Review.
-
Crosstalk between colorectal cancer cells and cancer-associated fibroblasts in the tumor microenvironment mediated by exosomal noncoding RNAs.Front Immunol. 2023 May 10;14:1161628. doi: 10.3389/fimmu.2023.1161628. eCollection 2023. Front Immunol. 2023. PMID: 37234178 Free PMC article. Review.
-
Serum-Soluble CD163 Levels as a Prognostic Biomarker in Patients with Diffuse Large B-Cell Lymphoma Treated with Chemoimmunotherapy.Int J Mol Sci. 2024 Mar 1;25(5):2862. doi: 10.3390/ijms25052862. Int J Mol Sci. 2024. PMID: 38474108 Free PMC article.
-
The future of immunotherapy for diffuse large B-cell lymphoma.Int J Cancer. 2025 Jan 15;156(2):251-261. doi: 10.1002/ijc.35156. Epub 2024 Sep 25. Int J Cancer. 2025. PMID: 39319495 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

