Dicoumarol: from chemistry to antitumor benefits
- PMID: 36575479
- PMCID: PMC9793554
- DOI: 10.1186/s13020-022-00699-0
Dicoumarol: from chemistry to antitumor benefits
Abstract
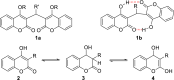
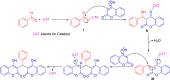
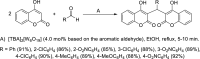
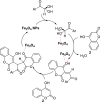
Dicoumarol, a coumarin-like compound, is known for its anticoagulant properties associated with the ability to inhibit vitamin K, being prescribed as a drug for several decades. The pharmaceutical value of dicoumarol turned it into a focus of chemists' attention, aiming its synthesis and of dicoumarol derivatives, bringing to light new methodologies. In recent years, several other bioactive effects have been claimed for dicoumarol and its derivatives, including anti-inflammatory, antimicrobial, antifungal, and anticancer, although the mechanisms of action underlying them are mostly not disclosed and additional research is needed to unravel them. This review presents a state of the art on the chemistry of dicoumarols, and their potential anticancer characteristics, highlighting the mechanisms of action elucidated so far. In parallel, we draw attention to the lack of in vivo studies and clinical trials to assess the safety and efficacy as drugs for later application.
Keywords: Anticancer; Biological activity; Coumarins; Dicoumarol; Synthesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
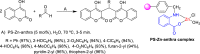


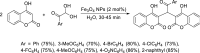
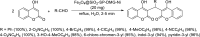



Similar articles
-
A pharmacological review of dicoumarol: An old natural anticoagulant agent.Pharmacol Res. 2020 Oct;160:105193. doi: 10.1016/j.phrs.2020.105193. Epub 2020 Sep 8. Pharmacol Res. 2020. PMID: 32911072 Review.
-
Dicoumarol: A Drug which Hits at Least Two Very Different Targets in Vitamin K Metabolism.Curr Drug Targets. 2017;18(5):500-510. doi: 10.2174/1389450116666150722141906. Curr Drug Targets. 2017. PMID: 26201483 Review.
-
Anticancer Potential of Coumarin and its Derivatives.Mini Rev Med Chem. 2021;21(19):2996-3029. doi: 10.2174/1389557521666210405160323. Mini Rev Med Chem. 2021. PMID: 33820507 Review.
-
Therapeutic Journey and Recent Advances in the Synthesis of Coumarin Derivatives.Mini Rev Med Chem. 2022;22(9):1314-1330. doi: 10.2174/1389557521666211116120823. Mini Rev Med Chem. 2022. PMID: 34784861 Review.
-
An insight into the therapeutic applications of coumarin compounds and their mechanisms of action.Eur J Pharm Sci. 2020 Sep 1;152:105424. doi: 10.1016/j.ejps.2020.105424. Epub 2020 Jun 10. Eur J Pharm Sci. 2020. PMID: 32534193 Review.
Cited by
-
Synthesis and Antiallergic Activity of Dicoumarin Derivatives.Molecules. 2024 Aug 10;29(16):3799. doi: 10.3390/molecules29163799. Molecules. 2024. PMID: 39202878 Free PMC article.
-
NQO1 Mediates Lenvatinib Resistance by Regulating ROS-induced Apoptosis in Hepatocellular Carcinoma.Curr Med Sci. 2024 Feb;44(1):168-179. doi: 10.1007/s11596-023-2804-8. Epub 2024 Jan 13. Curr Med Sci. 2024. PMID: 38217831
-
Unveiling the antitumor potential of novel N-(substituted-phenyl)-8-methoxycoumarin-3-carboxamides as dual inhibitors of VEGFR2 kinase and cytochrome P450 for targeted treatment of hepatocellular carcinoma.Front Chem. 2023 Aug 4;11:1231030. doi: 10.3389/fchem.2023.1231030. eCollection 2023. Front Chem. 2023. PMID: 37601910 Free PMC article.
-
Triethylammonium salt of a synthesized dicoumarol: Structural insight and human anti-glioblastoma activities.Heliyon. 2023 Jun 25;9(7):e17601. doi: 10.1016/j.heliyon.2023.e17601. eCollection 2023 Jul. Heliyon. 2023. PMID: 37456028 Free PMC article.
References
-
- Hussain MI, Syed QA, Khattak MNK, Hafez B, Reigosa MJ, El-Keblawy A. Natural product coumarins: biological and pharmacological perspectives. Biologia. 2019;74:863–888. doi: 10.2478/s11756-019-00242-x. - DOI
-
- Al-Majedy Y, Al-Amiery A, Kadhum AA, BakarMohamad A. Antioxidant activity of coumarins. Syst Rev Pharm. 2016;8:24–30. doi: 10.5530/srp.2017.1.6. - DOI
-
- Grover J, Jachak SM. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015;5:38892–38905. doi: 10.1039/C5RA05643H. - DOI
Publication types
LinkOut - more resources
Full Text Sources

