Parasite infections, neuroinflammation, and potential contributions of gut microbiota
- PMID: 36569929
- PMCID: PMC9772015
- DOI: 10.3389/fimmu.2022.1024998
Parasite infections, neuroinflammation, and potential contributions of gut microbiota
Abstract
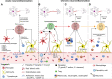
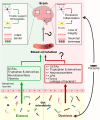
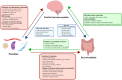
Many parasitic diseases (including cerebral malaria, human African trypanosomiasis, cerebral toxoplasmosis, neurocysticercosis and neuroschistosomiasis) feature acute or chronic brain inflammation processes, which are often associated with deregulation of glial cell activity and disruption of the brain blood barrier's intactness. The inflammatory responses of astrocytes and microglia during parasite infection are strongly influenced by a variety of environmental factors. Although it has recently been shown that the gut microbiota influences the physiology and immunomodulation of the central nervous system in neurodegenerative diseases like Alzheimer's disease and Parkinson's, the putative link in parasite-induced neuroinflammatory diseases has not been well characterized. Likewise, the central nervous system can influence the gut microbiota. In parasite infections, the gut microbiota is strongly perturbed and might influence the severity of the central nervous system inflammation response through changes in the production of bacterial metabolites. Here, we review the roles of astrocytes and microglial cells in the neuropathophysiological processes induced by parasite infections and their possible regulation by the gut microbiota.
Keywords: astrocytes; brain-inflammation; gut microbiota; immunopathophysiology; immunoregulation; microglia; parasitic disease.
Copyright © 2022 Alloo, Leleu, Grangette and Pied.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging.Front Immunol. 2022 Apr 7;13:796288. doi: 10.3389/fimmu.2022.796288. eCollection 2022. Front Immunol. 2022. PMID: 35464431 Free PMC article. Review.
-
The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system.J Neuroendocrinol. 2019 May;31(5):e12684. doi: 10.1111/jne.12684. Epub 2019 Feb 1. J Neuroendocrinol. 2019. PMID: 30614568 Review.
-
Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer's Disease.Nutrients. 2018 Nov 14;10(11):1765. doi: 10.3390/nu10111765. Nutrients. 2018. PMID: 30441866 Free PMC article. Review.
-
Modulation of Gut Microbiota Through Dietary Intervention in Neuroinflammation and Alzheimer's and Parkinson's Diseases.Curr Nutr Rep. 2024 Jun;13(2):82-96. doi: 10.1007/s13668-024-00539-7. Epub 2024 Apr 23. Curr Nutr Rep. 2024. PMID: 38652236 Free PMC article. Review.
-
Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer's disease.Mol Neurodegener. 2022 Mar 5;17(1):19. doi: 10.1186/s13024-022-00522-2. Mol Neurodegener. 2022. PMID: 35248147 Free PMC article. Review.
Cited by
-
Neuroinflammation in Alzheimer's Disease: A Potential Role of Nose-Picking in Pathogen Entry via the Olfactory System?Biomolecules. 2023 Oct 24;13(11):1568. doi: 10.3390/biom13111568. Biomolecules. 2023. PMID: 38002250 Free PMC article. Review.
-
Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections.Molecules. 2023 Jun 27;28(13):5032. doi: 10.3390/molecules28135032. Molecules. 2023. PMID: 37446699 Free PMC article. Review.
-
High-fat diet-induced L-saccharopine accumulation inhibits estradiol synthesis and damages oocyte quality by disturbing mitochondrial homeostasis.Gut Microbes. 2024 Jan-Dec;16(1):2412381. doi: 10.1080/19490976.2024.2412381. Epub 2024 Oct 16. Gut Microbes. 2024. PMID: 39410876 Free PMC article.
References
-
- Organisation mondiale de la santé . World malaria report 2018. (Geneva: World Health Organization; ) (2018).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

