The role of erythrocytes and erythroid progenitor cells in tumors
- PMID: 36567722
- PMCID: PMC9755711
- DOI: 10.1515/biol-2022-0102
The role of erythrocytes and erythroid progenitor cells in tumors
Abstract
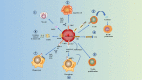
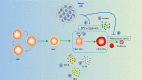
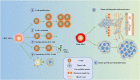
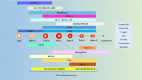
In the current research context of precision treatment of malignant tumors, the advantages of immunotherapy are unmatched by conventional antitumor therapy, which can prolong progression-free survival and overall survival. The search for new targets and novel combination therapies can improve the efficacy of immunotherapy and reduce adverse effects. Since current research targets for immunotherapy mainly focus on lymphocytes, little research has been done on erythrocytes. Nucleated erythroid precursor stem cells have been discovered to play an essential role in tumor progression. Researchers are exploring new targets and therapeutic approaches for immunotherapy from the perspective of erythroid progenitor cells (EPCs). Recent studies have shown that different subtypes of EPCs have specific surface markers and distinct biological roles in tumor immunity. CD45+ EPCs are potent myeloid-derived suppressor cell-like immunosuppressants that reduce the patient's antitumor immune response. CD45- EPCs promote tumor invasion and metastasis by secreting artemin. A specific type of EPC also promotes angiogenesis and provides radiation protection. Therefore, EPCs may be involved in tumor growth, infiltration, and metastasis. It may also be an important cause of anti-angiogenesis and immunotherapy resistance. This review summarizes recent research advances in erythropoiesis, EPC features, and their impacts and processes on tumors.
Keywords: EPCs; anemia; erythroid progenitor cells; immunotherapy; tumor.
© 2022 Hao Zhang et al., published by De Gruyter.
Conflict of interest statement
Conflict of interest: Authors state no conflict of interest.
Figures
Similar articles
-
Tumor Immune Evasion Induced by Dysregulation of Erythroid Progenitor Cells Development.Cancers (Basel). 2021 Feb 19;13(4):870. doi: 10.3390/cancers13040870. Cancers (Basel). 2021. PMID: 33669537 Free PMC article. Review.
-
Targeting erythroid progenitor cells for cancer immunotherapy.Int J Cancer. 2024 Dec 1;155(11):1928-1938. doi: 10.1002/ijc.35102. Epub 2024 Jul 22. Int J Cancer. 2024. PMID: 39039820 Review.
-
Tumor-associated CD8+T cell tolerance induced by erythroid progenitor cells.Front Immunol. 2024 May 10;15:1381919. doi: 10.3389/fimmu.2024.1381919. eCollection 2024. Front Immunol. 2024. PMID: 38799424 Free PMC article.
-
Understanding and targeting erythroid progenitor cells for effective cancer therapy.Curr Opin Hematol. 2023 Jul 1;30(4):137-143. doi: 10.1097/MOH.0000000000000762. Epub 2023 Apr 19. Curr Opin Hematol. 2023. PMID: 37052294 Free PMC article. Review.
-
Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells.Nat Med. 2018 Oct;24(10):1536-1544. doi: 10.1038/s41591-018-0205-5. Epub 2018 Oct 8. Nat Med. 2018. PMID: 30297899 Free PMC article.
Cited by
-
Identification and validation of ferroptosis related markers in erythrocyte differentiation of umbilical cord blood-derived CD34+ cell by bioinformatic analysis.Front Genet. 2024 Jul 30;15:1365232. doi: 10.3389/fgene.2024.1365232. eCollection 2024. Front Genet. 2024. PMID: 39139819 Free PMC article.
-
Importance of CD71+ Erythrocyte Cell Levels in Prognosis in Patients With β-Thalassemia.J Clin Lab Anal. 2024 Jun;38(11-12):e25084. doi: 10.1002/jcla.25084. Epub 2024 Jun 26. J Clin Lab Anal. 2024. PMID: 38924171 Free PMC article.
-
Exploring unconventional attributes of red blood cells and their potential applications in biomedicine.Protein Cell. 2024 May 7;15(5):315-330. doi: 10.1093/procel/pwae001. Protein Cell. 2024. PMID: 38270470 Free PMC article. No abstract available.
References
-
- Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021 Apr;11(4):933–59. - PubMed
-
- Chen J, Qiao YD, Li X, Xu JL, Ye QJ, Jiang N, et al. Intratumoral CD45+CD71+ erythroid cells induce immune tolerance and predict tumor recurrence in hepatocellular carcinoma. Cancer Lett. 2021 Feb 28;499:85–98. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
