Significance of Preexisting Vector Immunity and Activation of Innate Responses for Adenoviral Vector-Based Therapy
- PMID: 36560730
- PMCID: PMC9787786
- DOI: 10.3390/v14122727
Significance of Preexisting Vector Immunity and Activation of Innate Responses for Adenoviral Vector-Based Therapy
Abstract
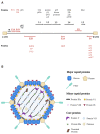
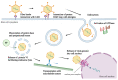
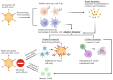
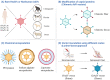
An adenoviral (AdV)-based vector system is a promising platform for vaccine development and gene therapy applications. Administration of an AdV vector elicits robust innate immunity, leading to the development of humoral and cellular immune responses against the vector and the transgene antigen, if applicable. The use of high doses (1011-1013 virus particles) of an AdV vector, especially for gene therapy applications, could lead to vector toxicity due to excessive levels of innate immune responses, vector interactions with blood factors, or high levels of vector transduction in the liver and spleen. Additionally, the high prevalence of AdV infections in humans or the first inoculation with the AdV vector result in the development of vector-specific immune responses, popularly known as preexisting vector immunity. It significantly reduces the vector efficiency following the use of an AdV vector that is prone to preexisting vector immunity. Several approaches have been developed to overcome this problem. The utilization of rare human AdV types or nonhuman AdVs is the primary strategy to evade preexisting vector immunity. The use of heterologous viral vectors, capsid modification, and vector encapsulation are alternative methods to evade vector immunity. The vectors can be optimized for clinical applications with comprehensive knowledge of AdV vector immunity, toxicity, and circumvention strategies.
Keywords: adenoviral gene therapy; adenoviral immunity; adenoviral innate immunity; adenoviral vector immunity; adenoviral vector vaccine; adenovirus capsid; adenovirus tropism; blood factor; circumvention of preexisting vector immunity; hepatic toxicity; preexisting immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Adenoviral vector immunity: its implications and circumvention strategies.Curr Gene Ther. 2011 Aug;11(4):307-20. doi: 10.2174/156652311796150372. Curr Gene Ther. 2011. PMID: 21453277 Free PMC article. Review.
-
Comparative Evaluation of the Vaccine Efficacies of Three Adenovirus-Based Vector Types in the Friend Retrovirus Infection Model.J Virol. 2019 Oct 15;93(21):e01155-19. doi: 10.1128/JVI.01155-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375593 Free PMC article.
-
Longevity of adenovirus vector immunity in mice and its implications for vaccine efficacy.Vaccine. 2018 Oct 29;36(45):6744-6751. doi: 10.1016/j.vaccine.2018.09.031. Epub 2018 Sep 25. Vaccine. 2018. PMID: 30266488 Free PMC article.
-
Circumventing antivector immunity: potential use of nonhuman adenoviral vectors.Hum Gene Ther. 2014 Apr;25(4):285-300. doi: 10.1089/hum.2013.228. Epub 2014 Mar 25. Hum Gene Ther. 2014. PMID: 24499174 Free PMC article. Review.
-
Current strategies and future directions for eluding adenoviral vector immunity.Curr Gene Ther. 2006 Apr;6(2):215-26. doi: 10.2174/156652306776359478. Curr Gene Ther. 2006. PMID: 16611043 Free PMC article. Review.
Cited by
-
Clinical Application of Adenovirus (AdV): A Comprehensive Review.Viruses. 2024 Jul 8;16(7):1094. doi: 10.3390/v16071094. Viruses. 2024. PMID: 39066256 Free PMC article. Review.
-
Updates and New Perspectives on Adenoviral Gene Therapy and Vaccine Vectors.Viruses. 2023 Feb 13;15(2):514. doi: 10.3390/v15020514. Viruses. 2023. PMID: 36851728 Free PMC article.
-
Safety and Immunogenicity of a ChAd155-Vectored Respiratory Syncytial Virus Vaccine in Infants 6-7 Months of age: A Phase 1/2 Randomized Trial.J Infect Dis. 2024 Jan 12;229(1):95-107. doi: 10.1093/infdis/jiad271. J Infect Dis. 2024. PMID: 37477875 Free PMC article. Clinical Trial.
-
Enhancement of mucosal innate and adaptive immunity following intranasal immunization of mice with a bovine adenoviral vector.Front Immunol. 2023 Nov 21;14:1305937. doi: 10.3389/fimmu.2023.1305937. eCollection 2023. Front Immunol. 2023. PMID: 38077380 Free PMC article.
-
Development of NP-Based Universal Vaccine for Influenza A Viruses.Vaccines (Basel). 2024 Feb 2;12(2):157. doi: 10.3390/vaccines12020157. Vaccines (Basel). 2024. PMID: 38400140 Free PMC article.
References
-
- Human Adenovirus Working Group. [(accessed on 23 April 2020)]. Available online: http://hadvwg.gmu.edu/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

