The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants
- PMID: 36557168
- PMCID: PMC9788111
- DOI: 10.3390/membranes12121261
The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants
Abstract
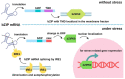
The membranes of plant cells are dynamic structures composed of phospholipids and proteins. Proteins harboring phospholipid-binding domains or lipid ligands can localize to membranes. Stress perception can alter the subcellular localization of these proteins dynamically, causing them to either associate with or detach from membranes. The mechanisms behind the re-localization involve changes in the lipidation state of the proteins and interactions with membrane-associated biomolecules. The functional significance of such re-localization includes the regulation of molecular transport, cell integrity, protein folding, signaling, and gene expression. In this review, proteins that re-localize to or away from membranes upon abiotic and biotic stresses will be discussed in terms of the mechanisms involved and the functional significance of their re-localization. Knowledge of the re-localization mechanisms will facilitate research on increasing plant stress adaptability, while the study on re-localization of proteins upon stresses will further our understanding of stress adaptation strategies in plants.
Keywords: co-translational modification; myristoylation; palmitoylation; plant stress adaptability; post-translational modification; prenylation; protein lipidation; protein re-localization; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nuclear Signaling of Plant MAPKs.Front Plant Sci. 2018 Apr 11;9:469. doi: 10.3389/fpls.2018.00469. eCollection 2018. Front Plant Sci. 2018. PMID: 29696029 Free PMC article. Review.
-
Combinatorial Interactions of Biotic and Abiotic Stresses in Plants and Their Molecular Mechanisms: Systems Biology Approach.Mol Biotechnol. 2018 Aug;60(8):636-650. doi: 10.1007/s12033-018-0100-9. Mol Biotechnol. 2018. PMID: 29943149 Review.
-
The Roles of CCCH Zinc-Finger Proteins in Plant Abiotic Stress Tolerance.Int J Mol Sci. 2021 Aug 3;22(15):8327. doi: 10.3390/ijms22158327. Int J Mol Sci. 2021. PMID: 34361093 Free PMC article. Review.
-
Current perspective on protein S-acylation in plants: more than just a fatty anchor?J Exp Bot. 2015 Mar;66(6):1599-606. doi: 10.1093/jxb/erv053. Epub 2015 Feb 27. J Exp Bot. 2015. PMID: 25725093 Review.
-
The importance of lipid modified proteins in plants.New Phytol. 2015 Jan;205(2):476-89. doi: 10.1111/nph.13085. Epub 2014 Oct 6. New Phytol. 2015. PMID: 25283240 Review.
Cited by
-
Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa.Molecules. 2023 May 27;28(11):4379. doi: 10.3390/molecules28114379. Molecules. 2023. PMID: 37298857 Free PMC article.
References
-
- Bhatnagar R.S., Ashrafi K., Fütterer K., Waksman G., Gordon J.I. Biology and enzymology of protein N-myristoylation. Enzymes. 2001;21:241–290.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources