The Insulin-like Growth Factor Signaling Pathway in Breast Cancer: An Elusive Therapeutic Target
- PMID: 36556357
- PMCID: PMC9782138
- DOI: 10.3390/life12121992
The Insulin-like Growth Factor Signaling Pathway in Breast Cancer: An Elusive Therapeutic Target
Abstract
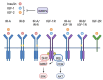
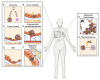
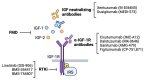
In this review, we provide an overview of the role of the insulin-like growth factor (IGF) signaling pathway in breast cancer and discuss its potential as a therapeutic target. The IGF pathway ligands, IGF-1 and IGF-2, and their receptors, primarily IGF-1R, are important for normal mammary gland biology, and dysregulation of their expression and function drives breast cancer risk and progression through activation of downstream signaling effectors, often in a subtype-dependent manner. The IGF signaling pathway has also been implicated in resistance to current therapeutic strategies, including ER and HER2 targeting drugs. Unfortunately, efforts to target IGF signaling for the treatment of breast cancer have been unsuccessful, due to a number of factors, most significantly the adverse effects of disrupting IGF signaling on normal glucose metabolism. We highlight here the recent discoveries that provide enthusiasm for continuing efforts to target IGF signaling for the treatment of breast cancer patients.
Keywords: IGF-1 receptor (IGF-1R); breast cancer; insulin receptor substrate (IRS) proteins; insulin-like growth factor (IGF); signal transduction; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Diversity of insulin and IGF signaling in breast cancer: Implications for therapy.Mol Cell Endocrinol. 2021 May 1;527:111213. doi: 10.1016/j.mce.2021.111213. Epub 2021 Feb 17. Mol Cell Endocrinol. 2021. PMID: 33607269 Free PMC article. Review.
-
Aiming for the Insulin-like Growth Factor-1 system in breast cancer therapeutics.Cancer Treat Rev. 2018 Feb;63:79-95. doi: 10.1016/j.ctrv.2017.11.010. Epub 2017 Dec 6. Cancer Treat Rev. 2018. PMID: 29253837 Review.
-
Inhibition of insulin-like growth factor signaling pathways in mammary gland by pure antiestrogen ICI 182,780.Clin Cancer Res. 2001 Aug;7(8):2545-54. Clin Cancer Res. 2001. PMID: 11489838
-
Compensatory role of insulin-like growth factor 1 receptor in estrogen receptor signaling pathway and possible therapeutic target for hormone therapy-resistant breast cancer.Breast Cancer. 2019 May;26(3):272-281. doi: 10.1007/s12282-018-0922-0. Epub 2018 Oct 16. Breast Cancer. 2019. PMID: 30328006
-
Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells.Cancer Res. 2001 Sep 15;61(18):6747-54. Cancer Res. 2001. PMID: 11559546
Cited by
-
Role of the Insulin-like Growth Factor (IGF) Axis in Diseases.Int J Mol Sci. 2023 Nov 30;24(23):16969. doi: 10.3390/ijms242316969. Int J Mol Sci. 2023. PMID: 38069291 Free PMC article.
-
Role of microRNAs in triple‑negative breast cancer and new therapeutic concepts (Review).Oncol Lett. 2024 Jul 11;28(3):431. doi: 10.3892/ol.2024.14565. eCollection 2024 Sep. Oncol Lett. 2024. PMID: 39049985 Free PMC article. Review.
-
Extracellular Interactors of the IGF System: Impact on Cancer Hallmarks and Therapeutic Approaches.Int J Mol Sci. 2024 May 29;25(11):5915. doi: 10.3390/ijms25115915. Int J Mol Sci. 2024. PMID: 38892104 Free PMC article. Review.
-
Decoding the Role of Insulin-like Growth Factor 1 and Its Isoforms in Breast Cancer.Int J Mol Sci. 2024 Aug 27;25(17):9302. doi: 10.3390/ijms25179302. Int J Mol Sci. 2024. PMID: 39273251 Free PMC article. Review.
-
Treating Alpelisib-Induced Hyperinsulinemia in Patients with Advanced Breast Cancer - A Real-Life Experience.Biologics. 2023 May 3;17:61-67. doi: 10.2147/BTT.S395817. eCollection 2023. Biologics. 2023. PMID: 37163178 Free PMC article.
References
-
- Frasca F., Pandini G., Scalia P., Sciacca L., Mineo R., Costantino A., Goldfine I.D., Belfiore A., Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999;19:3278–3288. doi: 10.1128/MCB.19.5.3278. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

