Cardiac Sarcomere Signaling in Health and Disease
- PMID: 36555864
- PMCID: PMC9782806
- DOI: 10.3390/ijms232416223
Cardiac Sarcomere Signaling in Health and Disease
Abstract
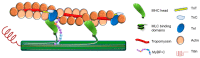
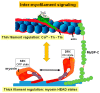
The cardiac sarcomere is a triumph of biological evolution wherein myriad contractile and regulatory proteins assemble into a quasi-crystalline lattice to serve as the central point upon which cardiac muscle contraction occurs. This review focuses on the many signaling components and mechanisms of regulation that impact cardiac sarcomere function. We highlight the roles of the thick and thin filament, both as necessary structural and regulatory building blocks of the sarcomere as well as targets of functionally impactful modifications. Currently, a new focus emerging in the field is inter-myofilament signaling, and we discuss here the important mediators of this mechanism, including myosin-binding protein C and titin. As the understanding of sarcomere signaling advances, so do the methods with which it is studied. This is reviewed here through discussion of recent live muscle systems in which the sarcomere can be studied under intact, physiologically relevant conditions.
Keywords: cardiac muscle; heart disease; myosin; myosin-binding protein c; sarcomere; titin; troponin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Sarcomere integrated biosensor detects myofilament-activating ligands in real time during twitch contractions in live cardiac muscle.J Mol Cell Cardiol. 2020 Oct;147:49-61. doi: 10.1016/j.yjmcc.2020.07.012. Epub 2020 Aug 11. J Mol Cell Cardiol. 2020. PMID: 32791214 Free PMC article.
-
Sarcomere length-dependent effects on Ca2+-troponin regulation in myocardium expressing compliant titin.J Gen Physiol. 2019 Jan 7;151(1):30-41. doi: 10.1085/jgp.201812218. Epub 2018 Dec 6. J Gen Physiol. 2019. PMID: 30523116 Free PMC article.
-
Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2306-11. doi: 10.1073/pnas.1516732113. Epub 2016 Feb 8. Proc Natl Acad Sci U S A. 2016. PMID: 26858417 Free PMC article.
-
Myofilaments: Movers and Rulers of the Sarcomere.Compr Physiol. 2017 Mar 16;7(2):675-692. doi: 10.1002/cphy.c160026. Compr Physiol. 2017. PMID: 28333386 Review.
-
Sarcomere mechanics in striated muscles: from molecules to sarcomeres to cells.Am J Physiol Cell Physiol. 2017 Aug 1;313(2):C134-C145. doi: 10.1152/ajpcell.00050.2017. Epub 2017 May 24. Am J Physiol Cell Physiol. 2017. PMID: 28539306 Review.
Cited by
-
Glycation in the cardiomyocyte.Vitam Horm. 2024;125:47-88. doi: 10.1016/bs.vh.2024.04.005. Epub 2024 May 24. Vitam Horm. 2024. PMID: 38997172 Review.
-
Extra-osseous Roles of the RANK-RANKL-OPG Axis with a Focus on Skeletal Muscle.Curr Osteoporos Rep. 2024 Dec;22(6):632-650. doi: 10.1007/s11914-024-00890-2. Epub 2024 Sep 26. Curr Osteoporos Rep. 2024. PMID: 39325366 Free PMC article. Review.
-
Focus on cardiac troponin complex: From gene expression to cardiomyopathy.Genes Dis. 2024 Mar 11;11(6):101263. doi: 10.1016/j.gendis.2024.101263. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39211905 Free PMC article. Review.
-
Biosafe cerium oxide nanozymes protect human pluripotent stem cells and cardiomyocytes from oxidative stress.J Nanobiotechnology. 2024 Mar 26;22(1):132. doi: 10.1186/s12951-024-02383-x. J Nanobiotechnology. 2024. PMID: 38532378 Free PMC article.
-
Nucleus Mechanosensing in Cardiomyocytes.Int J Mol Sci. 2023 Aug 28;24(17):13341. doi: 10.3390/ijms241713341. Int J Mol Sci. 2023. PMID: 37686151 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

