Overview of Anti-Inflammatory and Anti-Nociceptive Effects of Polyphenols to Halt Osteoarthritis: From Preclinical Studies to New Clinical Insights
- PMID: 36555503
- PMCID: PMC9779856
- DOI: 10.3390/ijms232415861
Overview of Anti-Inflammatory and Anti-Nociceptive Effects of Polyphenols to Halt Osteoarthritis: From Preclinical Studies to New Clinical Insights
Abstract
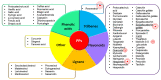
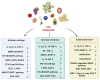
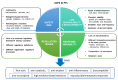
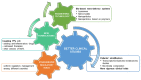
Knee osteoarthritis (OA) is one of the most multifactorial joint disorders in adults. It is characterized by degenerative and inflammatory processes that are responsible for joint destruction, pain and stiffness. Despite therapeutic advances, the search for alternative strategies to target inflammation and pain is still very challenging. In this regard, there is a growing body of evidence for the role of several bioactive dietary molecules (BDMs) in targeting inflammation and pain, with promising clinical results. BDMs may be valuable non-pharmaceutical solutions to treat and prevent the evolution of early OA to more severe phenotypes, overcoming the side effects of anti-inflammatory drugs. Among BDMs, polyphenols (PPs) are widely studied due to their abundance in several plants, together with their benefits in halting inflammation and pain. Despite their biological relevance, there are still many questionable aspects (biosafety, bioavailability, etc.) that hinder their clinical application. This review highlights the mechanisms of action and biological targets modulated by PPs, summarizes the data on their anti-inflammatory and anti-nociceptive effects in different preclinical in vitro and in vivo models of OA and underlines the gaps in the knowledge. Furthermore, this work reports the preliminary promising results of clinical studies on OA patients treated with PPs and discusses new perspectives to accelerate the translation of PPs treatment into the clinics.
Keywords: bioactive dietary molecules; inflammation; natural compounds; nutraceuticals; osteoarthritis; pain; polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies.Nutrients. 2021 Apr 23;13(5):1420. doi: 10.3390/nu13051420. Nutrients. 2021. PMID: 33922527 Free PMC article.
-
Apple polyphenols exhibits chondroprotective changes of synovium and prevents knee osteoarthritis.Biochem Biophys Res Commun. 2022 Jul 23;614:120-124. doi: 10.1016/j.bbrc.2022.05.016. Epub 2022 May 7. Biochem Biophys Res Commun. 2022. PMID: 35580540
-
A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models.BMC Vet Res. 2017 Aug 2;13(1):229. doi: 10.1186/s12917-017-1151-z. BMC Vet Res. 2017. PMID: 28768536 Free PMC article.
-
Gut Microbiota Modulation and Anti-Inflammatory Properties of Dietary Polyphenols in IBD: New and Consolidated Perspectives.Curr Pharm Des. 2017;23(16):2344-2351. doi: 10.2174/1381612823666170207145420. Curr Pharm Des. 2017. PMID: 28176667 Review.
-
Exploring the role of polyphenols in rheumatoid arthritis.Crit Rev Food Sci Nutr. 2022;62(19):5372-5393. doi: 10.1080/10408398.2021.1924613. Epub 2021 May 17. Crit Rev Food Sci Nutr. 2022. PMID: 33998910 Review.
Cited by
-
A standardized nutraceutical supplement contributes to pain relief, improves quality of life and regulates inflammation in knee osteoarthritis patients; A randomized clinical trial.Heliyon. 2023 Sep 14;9(9):e20143. doi: 10.1016/j.heliyon.2023.e20143. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809749 Free PMC article.
-
Epigenetic Modifications of MiRNAs in Osteoarthritis: A Systematic Review on Their Methylation Levels and Effects on Chondrocytes, Extracellular Matrix and Joint Inflammation.Cells. 2023 Jul 11;12(14):1821. doi: 10.3390/cells12141821. Cells. 2023. PMID: 37508486 Free PMC article. Review.
-
Protective and Pain-Killer Effects of AMC3, a Novel N-Formyl Peptide Receptors (FPRs) Modulator, in Experimental Models of Rheumatoid Arthritis.Antioxidants (Basel). 2023 Jun 2;12(6):1207. doi: 10.3390/antiox12061207. Antioxidants (Basel). 2023. PMID: 37371936 Free PMC article.
-
Insights into Osteogenesis Induced by Crude Brassicaceae Seeds Extracts: A Role for Glucosinolates.Nutrients. 2024 Oct 12;16(20):3457. doi: 10.3390/nu16203457. Nutrients. 2024. PMID: 39458452 Free PMC article.
References
-
- Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L., et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

