Expanding the Therapeutic Window of EGFR-Targeted PE24 Immunotoxin for EGFR-Overexpressing Cancers by Tailoring the EGFR Binding Affinity
- PMID: 36555466
- PMCID: PMC9779439
- DOI: 10.3390/ijms232415820
Expanding the Therapeutic Window of EGFR-Targeted PE24 Immunotoxin for EGFR-Overexpressing Cancers by Tailoring the EGFR Binding Affinity
Abstract
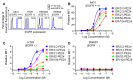
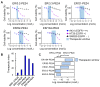
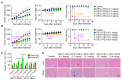
Immunotoxins (ITs), which are toxin-fused tumor antigen-specific antibody chimeric proteins, have been developed to selectively kill targeted cancer cells. The epidermal growth factor receptor (EGFR) is an attractive target for the development of anti-EGFR ITs against solid tumors due to its overexpression on the cell surface of various solid tumors. However, the low basal level expression of EGFR in normal tissue cells can cause undesirable on-target/off-tumor toxicity and reduce the therapeutic window of anti-EGFR ITs. Here, based on an anti-EGFR monobody with cross-reactivity to both human and murine EGFR, we developed a strategy to tailor the anti-EGFR affinity of the monobody-based ITs carrying a 24-kDa fragment of Pseudomonas exotoxin A (PE24), termed ER-PE24, to distinguish tumors that overexpress EGFR from normal tissues. Five variants of ER-PE24 were generated with different EGFR affinities (KD ≈ 0.24 nM to 104 nM), showing comparable binding activity for both human and murine EGFR. ER/0.2-PE24 with the highest affinity (KD ≈ 0.24 nM) exhibited a narrow therapeutic window of 19 pM to 93 pM, whereas ER/21-PE24 with an intermediate affinity (KD ≈ 21 nM) showed a much broader therapeutic window of 73 pM to 1.5 nM in in vitro cytotoxic assays using tumor model cell lines. In EGFR-overexpressing tumor xenograft mouse models, the maximum tolerated dose (MTD) of intravenous injection of ER/21-PE24 was found to be 0.4 mg/kg, which was fourfold higher than the MTD (0.1 mg/kg) of ER/0.2-PE24. Our study provides a strategy for the development of IT targeting tumor overexpressed antigens with basal expression in broad normal tissues by tailoring tumor antigen affinities.
Keywords: PE24 toxin; affinity variants; anti-EGFR monobody; immunotoxin; on-target/off-tumor toxicity; therapeutic index; therapeutic window.
Conflict of interest statement
The authors declare that they have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Enhancing the cytotoxicity of immunotoxins by facilitating their dissociation from target receptors under the reducing conditions of the endocytic pathway.Int J Biol Macromol. 2024 Oct;278(Pt 1):134668. doi: 10.1016/j.ijbiomac.2024.134668. Epub 2024 Aug 11. Int J Biol Macromol. 2024. PMID: 39137851
-
Highly Potent Immunotoxins Targeting the Membrane-distal N-lobe of GPC3 for Immunotherapy of Hepatocellular Carcinoma.J Cancer. 2022 Feb 14;13(4):1370-1384. doi: 10.7150/jca.66978. eCollection 2022. J Cancer. 2022. PMID: 35281879 Free PMC article.
-
Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A.Toxins (Basel). 2016 Jul 25;8(8):217. doi: 10.3390/toxins8080217. Toxins (Basel). 2016. PMID: 27463727 Free PMC article.
-
Immunotoxin Therapies for the Treatment of Epidermal Growth Factor Receptor-Dependent Cancers.Toxins (Basel). 2016 May 4;8(5):137. doi: 10.3390/toxins8050137. Toxins (Basel). 2016. PMID: 27153091 Free PMC article. Review.
-
Immunotoxins in cancer therapy: Review and update.Int Rev Immunol. 2017 Jul 4;36(4):207-219. doi: 10.1080/08830185.2017.1284211. Epub 2017 Mar 1. Int Rev Immunol. 2017. PMID: 28282218 Review.
Cited by
-
Special Issue "Bacterial Toxins and Cancer".Int J Mol Sci. 2024 Feb 9;25(4):2128. doi: 10.3390/ijms25042128. Int J Mol Sci. 2024. PMID: 38396805 Free PMC article.
-
Gamma radiation coupled ADP-ribosyl transferase activity of Pseudomonas aeruginosa PE24 moiety.Appl Microbiol Biotechnol. 2023 Mar;107(5-6):1765-1784. doi: 10.1007/s00253-023-12401-x. Epub 2023 Feb 18. Appl Microbiol Biotechnol. 2023. PMID: 36808279 Free PMC article.
-
Activation of the mTOR pathway enhances PPARγ/SREBP-mediated lipid synthesis in human meibomian gland epithelial cells.Sci Rep. 2024 Nov 15;14(1):28118. doi: 10.1038/s41598-024-73969-6. Sci Rep. 2024. PMID: 39548144 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

