A Study on the Protective Effect of sRAGE-MSCs in a Rodent Reperfusion Model of Myocardial Infarction
- PMID: 36555270
- PMCID: PMC9779272
- DOI: 10.3390/ijms232415630
A Study on the Protective Effect of sRAGE-MSCs in a Rodent Reperfusion Model of Myocardial Infarction
Abstract
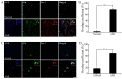
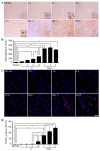
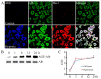
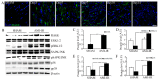
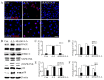
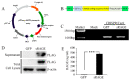
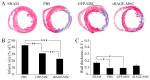
Acute myocardial infarction (AMI) is one of the major leading causes of death in humans globally. Recently, increased levels of recruited macrophages and AGE-albumin were observed in the hearts of humans and animals with acute myocardial infarction. Thus, the purposes of this study were to investigate whether the elevated levels of AGE-albumin from activated macrophage cells are implicated in ischemia-induced cardiomyocyte death and to develop therapeutic strategies for AMI based on its underlying molecular mechanisms with respect to AGEs. The present study demonstrated that activated macrophages and AGE-albumin were observed in heart tissues obtained from humans and rats with AMI incidences. In the cellular model of AMI, it was found that increased expression of AGE-albumin was shown to be co-localized with macrophages, and the presence of AGE-albumin led to increased expression of RAGE through the mitogen-activated protein kinase pathway. After revealing cardiomyocyte apoptosis induced by toxicity of the AGE-RAGE system, sRAGE-secreting MSCs were generated using the CRISPR/Cas9 platform to investigate the therapeutic effects of sRAGE-MSCs in an AMI rat model. Gene-edited sRAGE-MSCs showed greater therapeutic effects against AMI pathogenesis in rat models compared to mock MSCs, and promising results of the functional improvement of stem cells could result in significant improvements in the clinical management of cardiovascular diseases.
Keywords: AGE-albumin; MSCs; acute myocardial ischemia; cardiomyocyte death; gene editing; macrophage; soluble RAGE.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Advanced glycation end-product (AGE)-albumin from activated macrophage is critical in human mesenchymal stem cells survival and post-ischemic reperfusion injury.Sci Rep. 2017 Sep 14;7(1):11593. doi: 10.1038/s41598-017-11773-1. Sci Rep. 2017. PMID: 28912521 Free PMC article.
-
Soluble RAGE attenuates myocardial I/R injury by suppressing interleukin-6.Am J Med Sci. 2024 Aug 6:S0002-9629(24)01395-8. doi: 10.1016/j.amjms.2024.08.001. Online ahead of print. Am J Med Sci. 2024. PMID: 39111590
-
RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1823-32. doi: 10.1152/ajpheart.01210.2007. Epub 2008 Feb 1. Am J Physiol Heart Circ Physiol. 2008. PMID: 18245563
-
Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists.Mediators Inflamm. 2017;2017:7018393. doi: 10.1155/2017/7018393. Epub 2017 Feb 13. Mediators Inflamm. 2017. PMID: 28286377 Free PMC article. Review.
-
Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury.Cardiovasc Res. 2014 May 1;102(2):321-8. doi: 10.1093/cvr/cvu071. Epub 2014 Mar 27. Cardiovasc Res. 2014. PMID: 24675723 Review.
References
-
- Bayarsaikhan D., Bayarsaikhan G., Lee B. Recent advances in stem cells and gene editing: Drug discovery and therapeutics. Prog. Mol. Biol. Transl. Sci. 2021;181:231–269. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

