c-Abl Regulates the Pathological Deposition of TDP-43 via Tyrosine 43 Phosphorylation
- PMID: 36552734
- PMCID: PMC9776721
- DOI: 10.3390/cells11243972
c-Abl Regulates the Pathological Deposition of TDP-43 via Tyrosine 43 Phosphorylation
Abstract
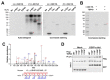
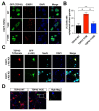
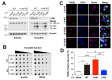
Non-receptor tyrosine kinase, c-Abl plays a role in the pathogenesis of several neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease. Here, we found that TDP-43, which was one of the main proteins comprising pathological deposits in amyotrophic lateral sclerosis (ALS), is a novel substrate for c-Abl. The phosphorylation of tyrosine 43 of TDP-43 by c-Abl led to increased TDP-43 levels in the cytoplasm and increased the formation of G3BP1-positive stress granules in SH-SY5Y cells. The kinase-dead mutant of c-Abl had no effect on the cytoplasmic localization of TDP-43. The expression of phosphor-mimetic mutant Y43E of TDP-43 in primary cortical neurons accumulated the neurite granule. Furthermore, the phosphorylation of TDP-43 at tyrosine 43 by c-Abl promoted the aggregation of TDP-43 and increased neuronal cell death in primary cortical neurons, but not in c-Abl-deficient primary cortical neurons. Identification of c-Abl as the kinase of TDP43 provides new insight into the pathogenesis of ALS.
Keywords: TDP-43; amyotrophic lateral sclerosis; c-Abl; mislocalization; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
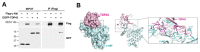

Similar articles
-
TDP-43 stabilizes G3BP1 mRNA: relevance to amyotrophic lateral sclerosis/frontotemporal dementia.Brain. 2021 Dec 16;144(11):3461-3476. doi: 10.1093/brain/awab217. Brain. 2021. PMID: 34115105 Free PMC article.
-
Abl kinase-mediated FUS Tyr526 phosphorylation alters nucleocytoplasmic FUS localization in FTLD-FUS.Brain. 2023 Oct 3;146(10):4088-4104. doi: 10.1093/brain/awad130. Brain. 2023. PMID: 37071594 Free PMC article.
-
Huntington's disease mice and human brain tissue exhibit increased G3BP1 granules and TDP43 mislocalization.J Clin Invest. 2021 Jun 15;131(12):e140723. doi: 10.1172/JCI140723. J Clin Invest. 2021. PMID: 33945510 Free PMC article.
-
The multi-functional RNA-binding protein G3BP1 and its potential implication in neurodegenerative disease.J Neurochem. 2021 May;157(4):944-962. doi: 10.1111/jnc.15280. Epub 2021 Jan 8. J Neurochem. 2021. PMID: 33349931 Free PMC article. Review.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
References
-
- Kabashi E., Valdmanis P.N., Dion P., Spiegelman D., McConkey B.J., Vande Velde C., Bouchard J.P., Lacomblez L., Pochigaeva K., Salachas F., et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. - DOI - PubMed
-
- Winton M.J., Igaz L.M., Wong M.M., Kwong L.K., Trojanowski J.Q., Lee V.M. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 2008;283:13302–13309. doi: 10.1074/jbc.M800342200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

