Transition-Metal-Free Difunctionalization of Sulfur Nucleophiles
- PMID: 36546892
- PMCID: PMC10108051
- DOI: 10.1002/anie.202216296
Transition-Metal-Free Difunctionalization of Sulfur Nucleophiles
Abstract
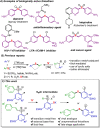
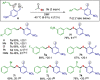
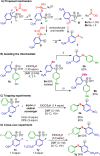
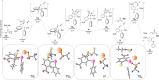
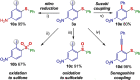
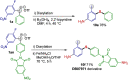
Efficient protocols for accessing iodo-substituted diaryl and aryl(vinyl) sulfides have been developed using iodonium salts as reactive electrophilic arylation and vinylation reagents. The reactions take place under transition-metal-free conditions, employing odorless and convenient sulfur reagents. A wide variety of functional groups are tolerated in the S-diarylation, enabling the regioselective late-stage application of several heterocycles and drug molecules under mild reaction conditions. A novel S-difunctionalization pathway was discovered using vinyliodonium salts, which proceeds under additive-free reaction conditions and grants excellent stereoselectivity in the synthesis of aryl(vinyl) sulfides. A one-pot strategy combining transition-metal-free diarylation and subsequent reduction provided facile access to electron-rich thioanilines and a direct synthesis of a potential drug candidate derivative. The retained iodo group allows a wide array of further synthetic transformations. Mechanistic insights were elucidated by isolating the key intermediate, and the relevant energy profile was substantiated by DFT calculations.
Keywords: Aromatic Substitution; Difunctionalization; Hypervalent Compounds; Iodonium Salts; Sulfides.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Electrophilic Vinylation of Thiols under Mild and Transition Metal-Free Conditions.Angew Chem Int Ed Engl. 2020 Sep 1;59(36):15512-15516. doi: 10.1002/anie.202002936. Epub 2020 Jun 15. Angew Chem Int Ed Engl. 2020. PMID: 32395880 Free PMC article.
-
Synthesis of Complex Diarylamines through a Ring-Opening Difunctionalization Strategy.Angew Chem Int Ed Engl. 2023 Nov 27;62(48):e202310921. doi: 10.1002/anie.202310921. Epub 2023 Oct 27. Angew Chem Int Ed Engl. 2023. PMID: 37847128
-
Iodine(III) Reagents in Radical Chemistry.Acc Chem Res. 2017 Jul 18;50(7):1712-1724. doi: 10.1021/acs.accounts.7b00148. Epub 2017 Jun 21. Acc Chem Res. 2017. PMID: 28636313 Free PMC article.
-
Aryl-, Akynyl-, and Alkenylbenziodoxoles: Synthesis and Synthetic Applications.Molecules. 2023 Feb 24;28(5):2136. doi: 10.3390/molecules28052136. Molecules. 2023. PMID: 36903382 Free PMC article. Review.
-
[Development of novel oxidation reactions using hypervalent iodine reagents and their application to total synthesis of biologically active natural products].Yakugaku Zasshi. 2000 Jul;120(7):620-9. doi: 10.1248/yakushi1947.120.7_620. Yakugaku Zasshi. 2000. PMID: 10920716 Review. Japanese.
Cited by
-
Ortho-ester-substituted diaryliodonium salts enabled regioselective arylocyclization of naphthols toward 3,4-benzocoumarins.Beilstein J Org Chem. 2024 Apr 18;20:841-851. doi: 10.3762/bjoc.20.76. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38655558 Free PMC article.
-
DBDMH-Promoted Methylthiolation in DMSO: A Metal-Free Protocol to Methyl Sulfur Compounds with Multifunctional Groups.Molecules. 2023 Jul 25;28(15):5635. doi: 10.3390/molecules28155635. Molecules. 2023. PMID: 37570605 Free PMC article.
-
Synthesis of Functionalized Pyrrolidinone Scaffolds via Smiles-Truce Cascade.Org Lett. 2023 Sep 15;25(36):6736-6740. doi: 10.1021/acs.orglett.3c02559. Epub 2023 Sep 5. Org Lett. 2023. PMID: 37668613 Free PMC article.
-
Synthetic exploration of electrophilic xanthylation via powerful N-xanthylphthalimides.Chem Sci. 2023 Aug 1;14(34):9197-9206. doi: 10.1039/d3sc03194b. eCollection 2023 Aug 30. Chem Sci. 2023. PMID: 37655020 Free PMC article.
-
Transition-Metal-Free C-Diarylations to Reach All-Carbon Quaternary Centers.JACS Au. 2024 Aug 5;4(8):2832-2837. doi: 10.1021/jacsau.4c00500. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211612 Free PMC article.
References
-
- Cremlyn R. J., Cremlyn R. J. W., An introduction to organosulfur chemistry, John Wiley & Sons, New York, 1996.
-
- None
-
- Hell R., Khan M. S., Wirtz M., in Cell Biology of Metals and Nutrients (Eds.: Hell R., Mendel R.-R.), Springer, Berlin, Heidelberg, 2010, pp. 43–279;
-
- Wang N., Saidhareddy P., Jiang X., Nat. Prod. Rep. 2020, 37, 246–275. - PubMed
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources

