DPY30 promotes the growth and survival of osteosarcoma cell by regulating the PI3K/AKT signal pathway
- PMID: 36546421
- PMCID: PMC9827427
- DOI: 10.4081/ejh.2023.3413
DPY30 promotes the growth and survival of osteosarcoma cell by regulating the PI3K/AKT signal pathway
Abstract
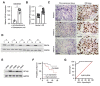
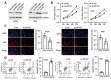
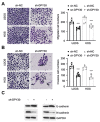
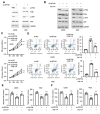
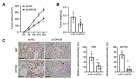
Osteosarcoma (OS) is characterized by aggressive features including invasiveness and high incidence of metastasis. OS patients with metastases are difficult to treat and suffer from a poor prognosis. DPY30 (protein dpy-30 homolog) is a key component of SET1/MLL family of H3K4 methyltransferases, which is implicated in the progression of multiple cancers. However, the potential functional engagement of DPY30 in OS remains to be unveiled. The objective of this study is to investigate the potential roles of DPY30 in the regulation of malignant phenotypes of OS cells. We examined DPY30 expression from a published dataset (GSE28424) as well as in OS tissues and adjacent normal tissues from OS patients. The association of DPY30 expression level and clinicopathologic parameters was assessed by Chi-square test. The role of DPY30 in regulating the malignant phenotype of OS cells and tumorigenesis was examined by in vitro functional assays and xenograft mouse model. We reported an upregulation of DPY30 in OS tumor tissues in both published dataset and clinical samples. A high level of DPY30 expression was associated with larger tumor size and more metastasis in OS patients, as well as poor overall survival. DPY30 knockdown in OS cells significantly impairs proliferation, migration and invasion, but induced cellular apoptosis. We further demonstrated that the agonist of PI3K/AKT pathway can rescue the inhibitory effects of DPY30 knockdown in OS cells. Together, our data indicate that DPY30 functions as an oncogene to promote the malignancy of OS cells possibly through PI3K/AKT pathway. The dependency of OS cells on DPY30 overexpression is a targetable vulnerability in OS cells.
Figures
Similar articles
-
HER4 promotes the growth and metastasis of osteosarcoma via the PI3K/AKT pathway.Acta Biochim Biophys Sin (Shanghai). 2020 Apr 20;52(4):345-362. doi: 10.1093/abbs/gmaa004. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 32181480
-
Knockdown of TBRG4 suppresses proliferation, invasion and promotes apoptosis of osteosarcoma cells by downregulating TGF-β1 expression and PI3K/AKT signaling pathway.Arch Biochem Biophys. 2020 Jun 15;686:108351. doi: 10.1016/j.abb.2020.108351. Epub 2020 Mar 30. Arch Biochem Biophys. 2020. PMID: 32240636
-
LncRNA LINC00628 overexpression inhibits the growth and invasion through regulating PI3K/Akt signaling pathway in osteosarcoma.Eur Rev Med Pharmacol Sci. 2018 Sep;22(18):5857-5866. doi: 10.26355/eurrev_201809_15915. Eur Rev Med Pharmacol Sci. 2018. PMID: 30280767
-
New emerging targets in osteosarcoma therapy: PTEN and PI3K/Akt crosstalk in carcinogenesis.Pathol Res Pract. 2023 Nov;251:154902. doi: 10.1016/j.prp.2023.154902. Epub 2023 Oct 21. Pathol Res Pract. 2023. PMID: 37922723 Review.
-
Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma.Open Life Sci. 2024 Aug 6;19(1):20220936. doi: 10.1515/biol-2022-0936. eCollection 2024. Open Life Sci. 2024. PMID: 39119480 Free PMC article. Review.
Cited by
-
A novel regulatory sex-skewing method that inhibits testicular DPY30 expression to increase female rate of dairy goat offspring.J Anim Sci. 2024 Jan 3;102:skad422. doi: 10.1093/jas/skad422. J Anim Sci. 2024. PMID: 38167777 Free PMC article.
-
DPY30 knockdown suppresses colorectal carcinoma progression via inducing Raf1/MST2-mediated apoptosis.Heliyon. 2024 Jan 20;10(3):e24807. doi: 10.1016/j.heliyon.2024.e24807. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38314299 Free PMC article.
References
-
- Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer 2014;14:722-35. - PubMed
-
- Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol 2021;18:609-24. - PubMed
-
- Kim W, Han I, Lee JS, Cho HS, Park JW, Kim H-S. Postmetastasis survival in high-grade extremity osteosarcoma: A retrospective analysis of prognostic factors in 126 patients. J Surg Oncol 2018;117:1223-31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

