Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma
- PMID: 36543907
- PMCID: PMC9886553
- DOI: 10.1038/s43018-022-00502-x
Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma
Abstract
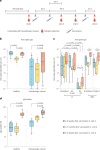
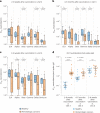
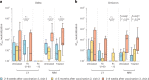
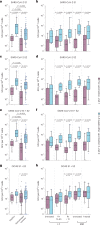
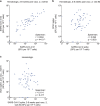
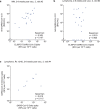
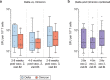
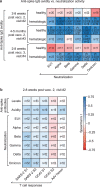
Individuals with hematologic malignancies are at increased risk for severe coronavirus disease 2019 (COVID-19), yet profound analyses of COVID-19 vaccine-induced immunity are scarce. Here we present an observational study with expanded methodological analysis of a longitudinal, primarily BNT162b2 mRNA-vaccinated cohort of 60 infection-naive individuals with B cell lymphomas and multiple myeloma. We show that many of these individuals, despite markedly lower anti-spike IgG titers, rapidly develop potent infection neutralization capacities against several severe acute respiratory syndrome coronavirus 2 variants of concern (VoCs). The observed increased neutralization capacity per anti-spike antibody unit was paralleled by an early step increase in antibody avidity between the second and third vaccination. All individuals with hematologic malignancies, including those depleted of B cells and individuals with multiple myeloma, exhibited a robust T cell response to peptides derived from the spike protein of VoCs Delta and Omicron (BA.1). Consistently, breakthrough infections were mainly of mild to moderate severity. We conclude that COVID-19 vaccination can induce broad antiviral immunity including ultrapotent neutralizing antibodies with high avidity in different hematologic malignancies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
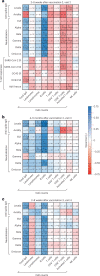
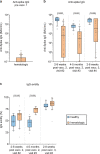
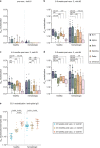
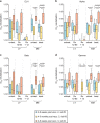

Comment in
-
Two approaches to tackling COVID-19 in patients with blood cancer.Nat Cancer. 2023 Jan;4(1):5-6. doi: 10.1038/s43018-022-00505-8. Nat Cancer. 2023. PMID: 36721072 No abstract available.
Similar articles
-
Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern.Nat Med. 2022 Mar;28(3):496-503. doi: 10.1038/s41591-022-01715-4. Epub 2022 Jan 28. Nat Med. 2022. PMID: 35090165
-
Antibody Response in Immunocompromised Patients With Hematologic Cancers Who Received a 3-Dose mRNA-1273 Vaccination Schedule for COVID-19.JAMA Oncol. 2022 Oct 1;8(10):1477-1483. doi: 10.1001/jamaoncol.2022.3227. JAMA Oncol. 2022. PMID: 35951338 Free PMC article.
-
NVX-CoV2373-induced cellular and humoral immunity towards parental SARS-CoV-2 and VOCs compared to BNT162b2 and mRNA-1273-regimens.J Clin Virol. 2022 Dec;157:105321. doi: 10.1016/j.jcv.2022.105321. Epub 2022 Oct 18. J Clin Virol. 2022. PMID: 36279695 Free PMC article.
-
Correlation of SARS-CoV-2 Viral Neutralizing Antibody Titers with Anti-Spike Antibodies and ACE-2 Inhibition among Vaccinated Individuals.Microbiol Spectr. 2022 Oct 26;10(5):e0131522. doi: 10.1128/spectrum.01315-22. Epub 2022 Sep 19. Microbiol Spectr. 2022. PMID: 36121252 Free PMC article.
-
Immune Response to COVID-19 Vaccination in Hematologic Malignancies: A Mini-Review.Chonnam Med J. 2023 Jan;59(1):24-30. doi: 10.4068/cmj.2023.59.1.24. Epub 2023 Jan 25. Chonnam Med J. 2023. PMID: 36794237 Free PMC article. Review.
Cited by
-
Clinical Features and Risk Stratification of Multiple Myeloma Patients with COVID-19.Cancers (Basel). 2023 Jul 13;15(14):3598. doi: 10.3390/cancers15143598. Cancers (Basel). 2023. PMID: 37509261 Free PMC article.
-
Relative Risk of Death in Bulgarian Cancer Patients during the Initial Waves of the COVID-19 Pandemic.Healthcare (Basel). 2023 Sep 20;11(18):2594. doi: 10.3390/healthcare11182594. Healthcare (Basel). 2023. PMID: 37761791 Free PMC article.
-
Live virus neutralizing antibodies against pre and post Omicron strains in food and retail workers in Québec, Canada.Heliyon. 2024 May 21;10(10):e31026. doi: 10.1016/j.heliyon.2024.e31026. eCollection 2024 May 30. Heliyon. 2024. PMID: 38826717 Free PMC article.
-
Cytokine-responsive T- and NK-cells portray SARS-CoV-2 vaccine-responders and infection in multiple myeloma patients.Leukemia. 2024 Jan;38(1):168-180. doi: 10.1038/s41375-023-02070-0. Epub 2023 Dec 4. Leukemia. 2024. PMID: 38049509 Free PMC article.
-
Immunologic Effect of Bivalent mRNA Booster in Patients Undergoing Hemodialysis.N Engl J Med. 2023 Mar 9;388(10):950-952. doi: 10.1056/NEJMc2216309. Epub 2023 Feb 15. N Engl J Med. 2023. PMID: 36791308 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

