Functional investigation of SLC1A2 variants associated with epilepsy
- PMID: 36543780
- PMCID: PMC9772344
- DOI: 10.1038/s41419-022-05457-6
Functional investigation of SLC1A2 variants associated with epilepsy
Abstract
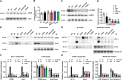
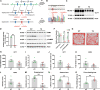
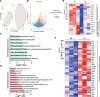
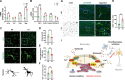
Epilepsy is a common neurological disorder and glutamate excitotoxicity plays a key role in epileptic pathogenesis. Astrocytic glutamate transporter GLT-1 is responsible for preventing excitotoxicity via clearing extracellular accumulated glutamate. Previously, three variants (G82R, L85P, and P289R) in SLC1A2 (encoding GLT-1) have been clinically reported to be associated with epilepsy. However, the functional validation and underlying mechanism of these GLT-1 variants in epilepsy remain undetermined. In this study, we reported that these disease-linked mutants significantly decrease glutamate uptake, cell membrane expression of the glutamate transporter, and glutamate-elicited current. Additionally, we found that these variants may disturbed stromal-interacting molecule 1 (STIM1)/Orai1-mediated store-operated Ca2+ entry (SOCE) machinery in the endoplasmic reticulum (ER), in which GLT-1 may be a new partner of SOCE. Furthermore, knock-in mice with disease-associated variants showed a hyperactive phenotype accompanied by reduced glutamate transporter expression. Therefore, GLT-1 is a promising and reliable therapeutic target for epilepsy interventions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Interplay between ER Ca2+ Binding Proteins, STIM1 and STIM2, Is Required for Store-Operated Ca2+ Entry.Int J Mol Sci. 2018 May 19;19(5):1522. doi: 10.3390/ijms19051522. Int J Mol Sci. 2018. PMID: 29783744 Free PMC article.
-
Filamin A Modulates Store-Operated Ca2+ Entry by Regulating STIM1 (Stromal Interaction Molecule 1)-Orai1 Association in Human Platelets.Arterioscler Thromb Vasc Biol. 2018 Feb;38(2):386-397. doi: 10.1161/ATVBAHA.117.310139. Epub 2017 Dec 28. Arterioscler Thromb Vasc Biol. 2018. PMID: 29284605
-
ORAI1/STIM1 Interaction Intervenes in Stroke and in Neuroprotection Induced by Ischemic Preconditioning Through Store-Operated Calcium Entry.Stroke. 2019 May;50(5):1240-1249. doi: 10.1161/STROKEAHA.118.024115. Stroke. 2019. PMID: 31009360
-
Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease.Cell Calcium. 2018 Dec;76:101-115. doi: 10.1016/j.ceca.2018.10.004. Epub 2018 Oct 30. Cell Calcium. 2018. PMID: 30414508 Free PMC article. Review.
-
The STIM-Orai Pathway: Conformational Coupling Between STIM and Orai in the Activation of Store-Operated Ca2+ Entry.Adv Exp Med Biol. 2017;993:83-98. doi: 10.1007/978-3-319-57732-6_5. Adv Exp Med Biol. 2017. PMID: 28900910 Free PMC article. Review.
Cited by
-
Single-cell sequencing of the substantia nigra reveals microglial activation in a model of MPTP.Front Aging Neurosci. 2024 Jun 17;16:1390310. doi: 10.3389/fnagi.2024.1390310. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38952478 Free PMC article.
-
In vitro human cell culture models in a bench-to-bedside approach to epilepsy.Epilepsia Open. 2024 Jun;9(3):865-890. doi: 10.1002/epi4.12941. Epub 2024 Apr 18. Epilepsia Open. 2024. PMID: 38637998 Free PMC article. Review.
-
GLT-1 downregulation in hippocampal astrocytes induced by type 2 diabetes contributes to postoperative cognitive dysfunction in adult mice.CNS Neurosci Ther. 2024 Sep;30(9):e70024. doi: 10.1111/cns.70024. CNS Neurosci Ther. 2024. PMID: 39218798 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

