Craniosynostosis, inner ear, and renal anomalies in a child with complete loss of SPRY1 (sprouty homolog 1) function
- PMID: 36543535
- PMCID: PMC10359576
- DOI: 10.1136/jmg-2022-108946
Craniosynostosis, inner ear, and renal anomalies in a child with complete loss of SPRY1 (sprouty homolog 1) function
Abstract
Introduction: SPRY1 encodes protein sprouty homolog 1 (Spry-1), a negative regulator of receptor tyrosine kinase signalling. Null mutant mice display kidney/urinary tract abnormalities and altered size of the skull; complete loss-of-function of Spry-1 in humans has not been reported.
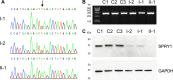
Methods: Analysis of whole-genome sequencing data from individuals with craniosynostosis enrolled in the 100,000 Genomes Project identified a likely pathogenic variant within SPRY1. Reverse-transcriptase PCR and western blot analysis were used to investigate the effect of the variant on SPRY1 mRNA and protein, in lymphoblastoid cell lines from the patient and both parents.
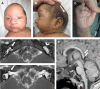
Results: A nonsense variant in SPRY1, encoding p.(Leu27*), was confirmed to be heterozygous in the unaffected parents and homozygous in the child. The child's phenotype, which included sagittal craniosynostosis, subcutaneous cystic lesions overlying the lambdoid sutures, hearing loss associated with bilateral cochlear and vestibular dysplasia and a unilateral renal cyst, overlapped the features reported in Spry1-/- null mice. Functional studies supported escape from nonsense-mediated decay, but western blot analysis demonstrated complete absence of full-length protein in the affected child and a marked reduction in both parents.
Conclusion: This is the first report of complete loss of Spry-1 function in humans, associated with abnormalities of the cranial sutures, inner ear, and kidneys.
Keywords: genetics; human genetics; musculoskeletal diseases.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Spry1 and Spry2 are necessary for eyelid closure.Dev Biol. 2013 Nov 15;383(2):227-38. doi: 10.1016/j.ydbio.2013.09.014. Epub 2013 Sep 17. Dev Biol. 2013. PMID: 24055172 Free PMC article.
-
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development.BMC Dev Biol. 2015 Oct 6;15:33. doi: 10.1186/s12861-015-0083-8. BMC Dev Biol. 2015. PMID: 26443994 Free PMC article.
-
Sprouty1 induces a senescence-associated secretory phenotype by regulating NFκB activity: implications for tumorigenesis.Cell Death Differ. 2014 Feb;21(2):333-43. doi: 10.1038/cdd.2013.161. Epub 2013 Nov 22. Cell Death Differ. 2014. PMID: 24270409 Free PMC article.
-
[An infant with premature closure of cranial sutures due to variant of ERF gene and a literature review].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2023 Aug 10;40(8):1009-1014. doi: 10.3760/cma.j.cn511374-20220815-00548. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2023. PMID: 37532503 Review. Chinese.
-
Sprouty proteins, masterminds of receptor tyrosine kinase signaling.Angiogenesis. 2008;11(1):53-62. doi: 10.1007/s10456-008-9089-1. Epub 2008 Jan 25. Angiogenesis. 2008. PMID: 18219583 Review.
Cited by
-
The value of genome-wide analysis in craniosynostosis.Front Genet. 2024 Jan 22;14:1322462. doi: 10.3389/fgene.2023.1322462. eCollection 2023. Front Genet. 2024. PMID: 38318288 Free PMC article.
-
Review of Recurrently Mutated Genes in Craniosynostosis Supports Expansion of Diagnostic Gene Panels.Genes (Basel). 2023 Feb 28;14(3):615. doi: 10.3390/genes14030615. Genes (Basel). 2023. PMID: 36980886 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
