Persistence of Mycobacterium tuberculosis in response to infection burden and host-induced stressors
- PMID: 36530432
- PMCID: PMC9755487
- DOI: 10.3389/fcimb.2022.981827
Persistence of Mycobacterium tuberculosis in response to infection burden and host-induced stressors
Abstract
Introduction: As infection with Mycobacterium tuberculosis progresses, the bacilli experience various degrees of host stressors in the macrophage phagosome such as low pH, nutrient deprivation, or exposure to toxic agents, which promotes cell-to-cell phenotypic variation. This includes a physiologically viable but non- or slowly replicating persister subpopulation, which is characterised by a loss of growth on solid media, while remaining metabolically active. Persisters additionally evade the host immune response and macrophage antimicrobial processes by adapting their metabolic pathways to maintain survival and persistence in the host.
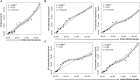
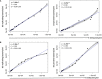
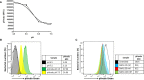
Methods: A flow cytometry-based dual-fluorescent replication reporter assay, termed fluorescence dilution, provided a culture-independent method to characterize the single-cell replication dynamics of M. tuberculosis persisters following macrophage infection. Fluorescence dilution in combination with reference counting beads and a metabolic esterase reactive probe, calcein violet AM, provided an effective approach to enumerate and characterize the phenotypic heterogeneity within M. tuberculosis following macrophage infection.
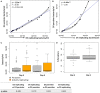
Results: Persister formation appeared dependent on the initial infection burden and intracellular bacterial burden. However, inhibition of phagocytosis by cytochalasin D treatment resulted in a significantly higher median percentage of persisters compared to inhibition of phagosome acidification by bafilomycin A1 treatment.
Discussion: Our results suggest that different host factors differentially impact the intracellular bacterial burden, adaptive mechanisms and entry into persistence in macrophages.
Keywords: Mycobacterium tuberculosis; bacterial heterogeneity; host-pathogen interaction; persistence; persisters; phagocytosis; phagosome acidification.
Copyright © 2022 Parbhoo, Schurz, Mouton and Sampson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Phenotypic adaptation of Mycobacterium tuberculosis to host-associated stressors that induce persister formation.Front Cell Infect Microbiol. 2022 Sep 27;12:956607. doi: 10.3389/fcimb.2022.956607. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36237425 Free PMC article. Review.
-
Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv.FEBS J. 2010 Jun;277(11):2416-27. doi: 10.1111/j.1742-4658.2010.07666.x. FEBS J. 2010. PMID: 20553485 Review.
-
Bioenergetic Heterogeneity in Mycobacterium tuberculosis Residing in Different Subcellular Niches.mBio. 2021 Jun 29;12(3):e0108821. doi: 10.1128/mBio.01088-21. Epub 2021 Jun 1. mBio. 2021. PMID: 34060333 Free PMC article.
-
Elucidating population-wide mycobacterial replication dynamics at the single-cell level.Microbiology (Reading). 2016 Jun;162(6):966-978. doi: 10.1099/mic.0.000288. Epub 2016 Mar 30. Microbiology (Reading). 2016. PMID: 27027532 Free PMC article.
-
Inside or outside the phagosome? The controversy of the intracellular localization of Mycobacterium tuberculosis.Tuberculosis (Edinb). 2012 Mar;92(2):113-20. doi: 10.1016/j.tube.2011.09.009. Epub 2011 Oct 26. Tuberculosis (Edinb). 2012. PMID: 22033468 Review.
Cited by
-
Understanding bacterial pathogenicity: a closer look at the journey of harmful microbes.Front Microbiol. 2024 Feb 20;15:1370818. doi: 10.3389/fmicb.2024.1370818. eCollection 2024. Front Microbiol. 2024. PMID: 38444801 Free PMC article. Review.
References
-
- Basu S., Pathak S. K., Banerjee A., Pathak S., Bhattacharyya A., Yang Z., et al. . (2007). Execution of macrophage apoptosis by PE_PGRS33 of mycobacterium tuberculosis is mediated by toll-like receptor 2-dependent release of tumor necrosis factor-α *. J. Biol. Chem. 282, 1039–1050. doi: 10.1074/jbc.M604379200 - DOI - PubMed
-
- Chengalroyen M. D., Beukes G. M., Gordhan B. G., Streicher E. M., Churchyard G., Hafner R., et al. . (2016). Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am. J. Respir. Crit. Care Med. 194, 1532–1540. doi: 10.1164/rccm.201604-0769OC - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

