Phenotypic and functional alterations of monocyte subsets with aging
- PMID: 36514074
- PMCID: PMC9745938
- DOI: 10.1186/s12979-022-00321-9
Phenotypic and functional alterations of monocyte subsets with aging
Abstract
Background: It has been widely accepted that monocytes are one of the central mediators contributing to inflammaging. However, it remains unclear whether aged monocytes, similar to aged T cells, have characteristics of hyperactivation and increased expression of co-inhibitory molecules.
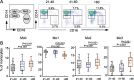
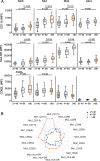
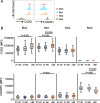
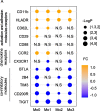
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from young (21-40 years old), middle-aged (41-60 years old), and older human subjects (> 60 years old). Flow cytometry was used to monitor changes in the expression of surface molecules of monocyte subsets and cytokine-producing capacity.
Results: We observed increased tumor necrosis factor-α: TNF-α and decreased interleukin-6 (IL-6) production in monocytes from older adults compared with young and middle-aged adults. Older adults had a greater percentage of intermediate and non-classical monocyte subsets, along with increased levels of the immune activation markers human leukocyte antigen-DR (HLA-DR), and adhesion molecules cluster of differentiation molecule 11b (CD11b) and L-selectin (CD62L). Furthermore, we observed increased C-C motif chemokine receptor 2 (CCR2) expression on classical monocytes and decreased C-X3-C motif chemokine receptor 1 (CX3CR1) expression on non-classical monocytes in older adult subjects. The expression of co-inhibitory receptors was reduced on monocyte subsets in older adults.
Conclusions: Circulating monocytes in older adults exhibit increased expression of activation, adhesion, and migration markers, but decreased expression of co-inhibitory molecules.
Keywords: Activation; Aging; Immunosenescence; Monocytes.
© 2022. The Author(s).
Conflict of interest statement
none.
Figures


Similar articles
-
Monocyte alteration in elderly hip fracture healing: monocyte promising role in bone regeneration.Immun Ageing. 2024 Feb 3;21(1):12. doi: 10.1186/s12979-024-00413-8. Immun Ageing. 2024. PMID: 38308312 Free PMC article. Review.
-
1,8-Cineol Attenuates Checkpoint Molecule PDL-1 and Adhesion Molecule CX3CR1 in Circulating Monocytes in Otitis Media Patients.J Pers Med. 2024 Mar 1;14(3):279. doi: 10.3390/jpm14030279. J Pers Med. 2024. PMID: 38541021 Free PMC article.
-
Monocyte subsets display age-dependent alterations at fasting and undergo non-age-dependent changes following consumption of a meal.Immun Ageing. 2022 Sep 14;19(1):41. doi: 10.1186/s12979-022-00297-6. Immun Ageing. 2022. PMID: 36104734 Free PMC article.
-
Differential expression of CCR2 and CX3CR1 on CD16+ monocyte subsets is associated with asthma severity.Allergy Asthma Clin Immunol. 2019 Nov 4;15:64. doi: 10.1186/s13223-019-0379-5. eCollection 2019. Allergy Asthma Clin Immunol. 2019. PMID: 31700522 Free PMC article.
-
Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis.Int Immunopharmacol. 2018 Dec;65:348-359. doi: 10.1016/j.intimp.2018.10.016. Epub 2018 Oct 23. Int Immunopharmacol. 2018. PMID: 30366278 Review.
Cited by
-
Impact of Immunosenescence in Older Kidney Transplant Recipients: Associated Clinical Outcomes and Possible Risk Stratification for Immunosuppression Reduction.Drugs Aging. 2024 Mar;41(3):219-238. doi: 10.1007/s40266-024-01100-5. Epub 2024 Feb 22. Drugs Aging. 2024. PMID: 38386164 Review.
-
Single and combined associations of blood lead and essential metals with serum lipid profiles in community-dwelling adults.Front Nutr. 2023 Apr 14;10:1129169. doi: 10.3389/fnut.2023.1129169. eCollection 2023. Front Nutr. 2023. PMID: 37125027 Free PMC article.
-
Age-associated changes in innate and adaptive immunity: role of the gut microbiota.Front Immunol. 2024 Sep 16;15:1421062. doi: 10.3389/fimmu.2024.1421062. eCollection 2024. Front Immunol. 2024. PMID: 39351234 Free PMC article. Review.
-
Hematopoietic aging promotes cancer by fueling IL-1⍺-driven emergency myelopoiesis.Science. 2024 Oct 25;386(6720):eadn0327. doi: 10.1126/science.adn0327. Epub 2024 Oct 25. Science. 2024. PMID: 39236155 Free PMC article.
-
Monocyte alteration in elderly hip fracture healing: monocyte promising role in bone regeneration.Immun Ageing. 2024 Feb 3;21(1):12. doi: 10.1186/s12979-024-00413-8. Immun Ageing. 2024. PMID: 38308312 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

