Intracellular Citrate/acetyl-CoA flux and endoplasmic reticulum acetylation: Connectivity is the answer
- PMID: 36513219
- PMCID: PMC9792894
- DOI: 10.1016/j.molmet.2022.101653
Intracellular Citrate/acetyl-CoA flux and endoplasmic reticulum acetylation: Connectivity is the answer
Abstract
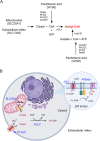
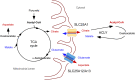
Background: Key cellular metabolites reflecting the immediate activity of metabolic enzymes as well as the functional metabolic state of intracellular organelles can act as powerful signal regulators to ensure the activation of homeostatic responses. The citrate/acetyl-CoA pathway, initially recognized for its role in intermediate metabolism, has emerged as a fundamental branch of this nutrient-sensing homeostatic response. Emerging studies indicate that fluctuations in acetyl-CoA availability within different cellular organelles and compartments provides substrate-level regulation of many biological functions. A fundamental aspect of these regulatory functions involves Nε-lysine acetylation.
Scope of review: Here, we will examine the emerging regulatory functions of the citrate/acetyl-CoA pathway and the specific role of the endoplasmic reticulum (ER) acetylation machinery in the maintenance of intracellular crosstalk and homeostasis. These functions will be analyzed in the context of associated human diseases and specific mouse models of dysfunctional ER acetylation and citrate/acetyl-CoA flux. A primary objective of this review is to highlight the complex yet integrated response of compartment- and organelle-specific Nε-lysine acetylation to the intracellular availability and flux of acetyl-CoA, linking this important post-translational modification to cellular metabolism.
Major conclusions: The ER acetylation machinery regulates the proteostatic functions of the organelle as well as the metabolic crosstalk between different intracellular organelles and compartments. This crosstalk enables the cell to impart adaptive responses within the ER and the secretory pathway. However, it also enables the ER to impart adaptive responses within different cellular organelles and compartments. Defects in the homeostatic balance of acetyl-CoA flux and ER acetylation reflect different but converging disease states in humans as well as converging phenotypes in relevant mouse models. In conclusion, citrate and acetyl-CoA should not only be seen as metabolic substrates of intermediate metabolism but also as signaling molecules that direct functional adaptation of the cell to both intracellular and extracellular messages. Future discoveries in CoA biology and acetylation are likely to yield novel therapeutic approaches.
Keywords: Acetyl-CoA; Acetylation; Citrate; CoA; Endoplasmic reticulum.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures

Similar articles
-
The citrate transporters SLC13A5 and SLC25A1 elicit different metabolic responses and phenotypes in the mouse.Commun Biol. 2023 Sep 9;6(1):926. doi: 10.1038/s42003-023-05311-1. Commun Biol. 2023. PMID: 37689798 Free PMC article.
-
Acetyl-CoA flux regulates the proteome and acetyl-proteome to maintain intracellular metabolic crosstalk.Nat Commun. 2019 Sep 2;10(1):3929. doi: 10.1038/s41467-019-11945-9. Nat Commun. 2019. PMID: 31477734 Free PMC article.
-
Nε-lysine acetylation in the endoplasmic reticulum - a novel cellular mechanism that regulates proteostasis and autophagy.J Cell Sci. 2018 Nov 16;131(22):jcs221747. doi: 10.1242/jcs.221747. J Cell Sci. 2018. PMID: 30446507 Free PMC article. Review.
-
N-acetylation of secreted proteins in Apicomplexa is widespread and is independent of the ER acetyl-CoA transporter AT1.J Cell Sci. 2022 Aug 1;135(15):jcs259811. doi: 10.1242/jcs.259811. Epub 2022 Aug 5. J Cell Sci. 2022. PMID: 35621049
-
Protein acetylation and acetyl coenzyme a metabolism in budding yeast.Eukaryot Cell. 2014 Dec;13(12):1472-83. doi: 10.1128/EC.00189-14. Epub 2014 Oct 17. Eukaryot Cell. 2014. PMID: 25326522 Free PMC article. Review.
Cited by
-
Ca+2 and Nε-lysine acetylation regulate the CALR-ATG9A interaction in the lumen of the endoplasmic reticulum.Sci Rep. 2024 Oct 26;14(1):25532. doi: 10.1038/s41598-024-76854-4. Sci Rep. 2024. PMID: 39462136 Free PMC article.
-
The citrate transporters SLC13A5 and SLC25A1 elicit different metabolic responses and phenotypes in the mouse.Commun Biol. 2023 Sep 9;6(1):926. doi: 10.1038/s42003-023-05311-1. Commun Biol. 2023. PMID: 37689798 Free PMC article.
-
Pyruvate Dehydrogenase-Dependent Metabolic Programming Affects the Oligodendrocyte Maturation and Remyelination.Mol Neurobiol. 2024 Jan;61(1):397-410. doi: 10.1007/s12035-023-03546-x. Epub 2023 Aug 24. Mol Neurobiol. 2024. PMID: 37620688
-
Spatial selectivity of ATase inhibition in mouse models of Charcot-Marie-Tooth disease.Brain Commun. 2024 Jul 9;6(4):fcae232. doi: 10.1093/braincomms/fcae232. eCollection 2024. Brain Commun. 2024. PMID: 39035418 Free PMC article.
-
Huppke-Brendel syndrome: Novel cases and a therapeutic trial with ketogenic diet and N-acetylcysteine.JIMD Rep. 2024 Jul 19;65(6):361-370. doi: 10.1002/jmd2.12439. eCollection 2024 Nov. JIMD Rep. 2024. PMID: 39512429 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

