Tumor necrosis factor-alpha blockade suppresses BK polyomavirus replication
- PMID: 36512270
- PMCID: PMC9745287
- DOI: 10.1007/s15010-022-01962-0
Tumor necrosis factor-alpha blockade suppresses BK polyomavirus replication
Abstract
Purpose: BK Polyomavirus (BKPyV) infection manifests as renal inflammation and can cause kidney damage. Tumor necrosis factor-α (TNF-α) is increased in renal inflammation and injury. The aim of this study was to investigate the effect of TNF-α blockade on BKPyV infection.
Methods: Urine specimens from 22 patients with BKPyV-associated nephropathy (BKPyVN) and 35 non-BKPyVN kidney transplant recipients were analyzed.
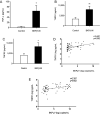
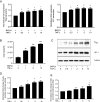
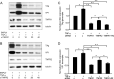
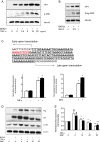
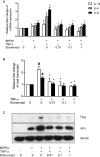
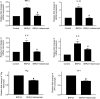
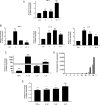
Results: We demonstrated increased urinary levels of TNF-α and its receptors, TNFR1 and TNFR2, in BKPyVN patients. Treating BKPyV-infected human proximal tubular cells (HRPTECs) with TNF-α stimulated the expression of large T antigen and viral capsid protein-1 mRNA and proteins and BKPyV promoter activity. Knockdown of TNFR1 or TNFR2 expression caused a reduction in TNF-α-stimulated viral replication. NF-κB activation induced by overexpression of constitutively active IKK2 significantly increased viral replication and the activity of the BKPyV promoter containing an NF-κB binding site. The addition of a NF-κB inhibitor on BKPyV-infected cells suppressed viral replication. Blockade of TNF-α functionality by etanercept reduced BKPyV-stimulated expression of TNF-α, interleukin-1β (IL-1β), IL-6 and IL-8 and suppressed TNF-α-stimulated viral replication. In cultured HRPTECs and THP-1 cells, BKPyV infection led to increased expression of TNF-α, interleukin-1 β (IL-1β), IL-6 and TNFR1 and TNFR2 but the stimulated magnitude was far less than that induced by poly(I:C). This may suggest that BKPyV-mediated autocrine effect is not a major source of TNFα.
Conclusion: TNF-α stimulates BKPyV replication and inhibition of its signal cascade or functionality attenuates its stimulatory effect. Our study provides a therapeutic anti-BKPyV target.
Keywords: BK polyomavirus; BKPyV-associated nephropathy; Large T antigen; Nuclear factor-κB; Tumor necrosis factor-α.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany.
Conflict of interest statement
All authors declare no conflict of interest. No financial or non-financial interests are directly or indirectly related to the work.
Figures
Similar articles
-
BK virus infection activates the TNFα/TNF receptor system in Polyomavirus-associated nephropathy.Mol Cell Biochem. 2016 Jan;411(1-2):191-9. doi: 10.1007/s11010-015-2581-1. Epub 2015 Oct 7. Mol Cell Biochem. 2016. PMID: 26446017
-
BK Polyomavirus Activates the DNA Damage Response To Prolong S Phase.J Virol. 2019 Jun 28;93(14):e00130-19. doi: 10.1128/JVI.00130-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043526 Free PMC article.
-
Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation.Circulation. 2009 Mar 17;119(10):1386-97. doi: 10.1161/CIRCULATIONAHA.108.802918. Epub 2009 Mar 2. Circulation. 2009. PMID: 19255345 Free PMC article.
-
The human polyomavirus BK (BKPyV): virological background and clinical implications.APMIS. 2013 Aug;121(8):728-45. doi: 10.1111/apm.12134. Epub 2013 Jun 19. APMIS. 2013. PMID: 23782063 Review.
-
Diagnostics, treatment, and immune response in BK polyomavirus infection after pediatric kidney transplantation.Pediatr Nephrol. 2020 Mar;35(3):375-382. doi: 10.1007/s00467-018-4164-3. Epub 2018 Dec 11. Pediatr Nephrol. 2020. PMID: 30539254 Review.
References
-
- Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136–145. doi: 10.1111/j.1600-6143.2012.04320.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

