GLUT-1/PKM2 loop dysregulation in patients with non-ST-segment elevation myocardial infarction promotes metainflammation
- PMID: 36508576
- PMCID: PMC10730239
- DOI: 10.1093/cvr/cvac184
GLUT-1/PKM2 loop dysregulation in patients with non-ST-segment elevation myocardial infarction promotes metainflammation
Abstract
Aims: The functional capacity of the immune cells is strongly dependent on their metabolic state and inflammatory responses are characterized by a greater use of glucose in immune cells. This study is aimed to establish the role of glucose metabolism and its players [glucose transporter 1 (GLUT-1) and pyruvate kinase isozyme M2 (PKM2)] in the dysregulation of adaptive immunity and inflammation observed in patients with non-ST-segment elevation myocardial infarction (NSTEMI).
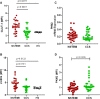
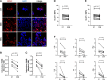
Methods and results: We enrolled 248 patients allocated to three groups: NSTEMI patients, chronic coronary syndromes (CCS) patients, healthy subjects (HSs). NSTEMI patients showed higher expression of GLUT-1 and an enhanced glucose uptake in T cells when compared with CCS patients (P < 0.0001; P = 0.0101, respectively) and HSs (P = 0.0071; P = 0.0122, respectively). PKM2 had a prevalent nuclear localization in T lymphocytes in NSTEMI (P = 0.0005 for nuclear vs. cytoplasm localization), while in CCS and HS, it was equally distributed in both compartments. In addition, the nuclear fraction of PKM2 was significantly higher in NSTEMI compared with HS (P = 0.0023). In NSTEMI patients, treatment with Shikonin and Fasentin, which inhibits PKM2 enzyme activity and GLUT-1-mediated glucose internalization, respectively, led to a significant reduction in GLUT-1 expression along with the down-regulation of pro-inflammatory cytokine expression.
Conclusion: NSTEMI patients exhibit dysregulation of the GLUT-1/PKM2 metabolic loop characterized by nuclear translocation of PKM2, where it acts as a transcription regulator of pro-inflammatory genes. This detrimental loop might represent a new therapeutic target for personalized medicine.
Keywords: Acute coronary syndromes; Adaptive immunity; GLUT-1; Immuno-metabolism; Meta-inflammation; PKM2; Precision medicine.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: F.Cr. reports speaker fees from Amgen, Astra Zeneca, Servier, BMS, other from GlyCardial Diagnostics, outside the submitted work. G.L. received grant support (to the institution) for investigator-initiated research from American Heart Association, Italian National Health Service, and Italian Minister of Education, University and Research. She is currently involved in the Research Programs of the Italian Cardiovascular Network. She received personal fees from Astra Zeneca, Boehringer Ingelheim, Novo Nordisk, Daiichi Sankyo, Sanofi, and Novartis, outside the submitted work.
Figures

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
A multicomponent psychosocial intervention to reduce substance use by adolescents involved in the criminal justice system: the RISKIT-CJS RCT.Public Health Res (Southampt). 2023 Mar;11(3):1-77. doi: 10.3310/FKPY6814. Public Health Res (Southampt). 2023. PMID: 37254608
-
Continuing education meetings and workshops: effects on professional practice and healthcare outcomes.Cochrane Database Syst Rev. 2021 Sep 15;9(9):CD003030. doi: 10.1002/14651858.CD003030.pub3. Cochrane Database Syst Rev. 2021. PMID: 34523128 Free PMC article. Review.
Cited by
-
Systemic and local vascular inflammation and arterial reactive oxygen species generation in patients with advanced cardiovascular diseases.Front Cardiovasc Med. 2023 Sep 7;10:1230051. doi: 10.3389/fcvm.2023.1230051. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37745103 Free PMC article.
-
Targeting Collagen Pathways as an HFpEF Therapeutic Strategy.J Clin Med. 2023 Sep 9;12(18):5862. doi: 10.3390/jcm12185862. J Clin Med. 2023. PMID: 37762803 Free PMC article. Review.
-
Meta-Inflammation and New Anti-Diabetic Drugs: A New Chance to Knock Down Residual Cardiovascular Risk.Int J Mol Sci. 2023 May 12;24(10):8643. doi: 10.3390/ijms24108643. Int J Mol Sci. 2023. PMID: 37239990 Free PMC article. Review.
-
The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches.Basic Res Cardiol. 2023 Nov 8;118(1):48. doi: 10.1007/s00395-023-01018-w. Basic Res Cardiol. 2023. PMID: 37938421 Free PMC article. Review.
-
Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways.Biology (Basel). 2024 Jul 12;13(7):519. doi: 10.3390/biology13070519. Biology (Basel). 2024. PMID: 39056712 Free PMC article. Review.
References
-
- Flego D, Severino A, Trotta F, Previtero M, Ucci S, Zara C, Massaro G, Pedicino D, Biasucci LM, Liuzzo G, Crea F. Increased PTPN22 expression and defective CREB activation impair regulatory T-cell differentiation in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2015;65:1175–1186. - PubMed
-
- Biasucci LM, La Rosa G, Pedicino D, D'Aiello A, Galli M, Liuzzo G. Where does inflammation fit? Curr Cardiol Rep 2017;19:84. - PubMed
-
- Angelini G, Flego D, Vinci R, Pedicino D, Trotta F, Ruggio A, Piemontese GP, Galante D, Ponzo M, Biasucci LM, Liuzzo G, Crea F. Matrix metalloproteinase-9 might affect adaptive immunity in non-ST segment elevation acute coronary syndromes by increasing CD31 cleavage on CD4+ T-cells. Eur Heart J 2018;39:1089–1097. - PMC - PubMed
-
- Ruggio A, Pedicino D, Flego D, Vergallo R, Severino A, Lucci C, Niccoli G, Trani C, Burzotta F, Aurigemma C, Leone AM, Buffon A, D'Aiello A, Biasucci LM, Crea F, Liuzzo G. Correlation between CD4(+)CD28(null) T lymphocytes, regulatory T cells and plaque rupture: an optical coherence tomography study in acute coronary syndromes. Int J Cardiol 2019;276:289–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

