Quorum sensing in human gut and food microbiomes: Significance and potential for therapeutic targeting
- PMID: 36504831
- PMCID: PMC9733432
- DOI: 10.3389/fmicb.2022.1002185
Quorum sensing in human gut and food microbiomes: Significance and potential for therapeutic targeting
Abstract
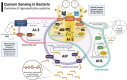
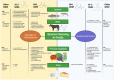
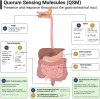
Human gut and food microbiomes interact during digestion. The outcome of these interactions influences the taxonomical composition and functional capacity of the resident human gut microbiome, with potential consequential impacts on health and disease. Microbe-microbe interactions between the resident and introduced microbiomes, which likely influence host colonisation, are orchestrated by environmental conditions, elements of the food matrix, host-associated factors as well as social cues from other microorganisms. Quorum sensing is one example of a social cue that allows bacterial communities to regulate genetic expression based on their respective population density and has emerged as an attractive target for therapeutic intervention. By interfering with bacterial quorum sensing, for instance, enzymatic degradation of signalling molecules (quorum quenching) or the application of quorum sensing inhibitory compounds, it may be possible to modulate the microbial composition of communities of interest without incurring negative effects associated with traditional antimicrobial approaches. In this review, we summarise and critically discuss the literature relating to quorum sensing from the perspective of the interactions between the food and human gut microbiome, providing a general overview of the current understanding of the prevalence and influence of quorum sensing in this context, and assessing the potential for therapeutic targeting of quorum sensing mechanisms.
Keywords: food matrix; food microbiome; gut microbiome; quorum quenching; quorum sensing; quorum sensing inhibition.
Copyright © 2022 Falà, Álvarez-Ordóñez, Filloux, Gahan and Cotter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The organophosphate malathion disturbs gut microbiome development and the quorum-Sensing system.Toxicol Lett. 2018 Feb;283:52-57. doi: 10.1016/j.toxlet.2017.10.023. Epub 2017 Oct 31. Toxicol Lett. 2018. PMID: 29097220
-
Modulation of Gut Microbiota through Dietary Phytochemicals as a Novel Anti-infective Strategy.Curr Drug Discov Technol. 2020;17(4):498-506. doi: 10.2174/1570163816666191107124214. Curr Drug Discov Technol. 2020. PMID: 31702513 Review.
-
Importance of N-Acyl-Homoserine Lactone-Based Quorum Sensing and Quorum Quenching in Pathogen Control and Plant Growth Promotion.Pathogens. 2021 Nov 30;10(12):1561. doi: 10.3390/pathogens10121561. Pathogens. 2021. PMID: 34959516 Free PMC article.
-
Quorum Sensing and Quorum Quenching in the Phycosphere of Phytoplankton: a Case of Chemical Interactions in Ecology.J Chem Ecol. 2016 Dec;42(12):1201-1211. doi: 10.1007/s10886-016-0791-y. Epub 2016 Nov 7. J Chem Ecol. 2016. PMID: 27822708 Review.
-
Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: a review of recent advances.Expert Rev Anti Infect Ther. 2020 Dec;18(12):1221-1233. doi: 10.1080/14787210.2020.1794815. Epub 2020 Aug 4. Expert Rev Anti Infect Ther. 2020. PMID: 32749905 Free PMC article. Review.
Cited by
-
Oral Cardiac Drug-Gut Microbiota Interaction in Chronic Heart Failure Patients: An Emerging Association.Int J Mol Sci. 2024 Jan 31;25(3):1716. doi: 10.3390/ijms25031716. Int J Mol Sci. 2024. PMID: 38338995 Free PMC article. Review.
-
The Interplay between Gut Microbiota and Oral Medications and Its Impact on Advancing Precision Medicine.Metabolites. 2023 May 21;13(5):674. doi: 10.3390/metabo13050674. Metabolites. 2023. PMID: 37233715 Free PMC article. Review.
-
Comprehensive insight into the alterations in the gut microbiome and the intestinal barrier as a consequence of iron deficiency anaemia.Biomed J. 2024 Dec;47(6):100701. doi: 10.1016/j.bj.2024.100701. Epub 2024 Jan 26. Biomed J. 2024. PMID: 38281699 Free PMC article.
-
Gut microbial metabolic signatures in diabetes mellitus and potential preventive and therapeutic applications.Gut Microbes. 2024 Jan-Dec;16(1):2401654. doi: 10.1080/19490976.2024.2401654. Epub 2024 Oct 18. Gut Microbes. 2024. PMID: 39420751 Free PMC article. Review.
-
Anti-Quorum Sensing Activity of Probiotics: The Mechanism and Role in Food and Gut Health.Microorganisms. 2023 Mar 20;11(3):793. doi: 10.3390/microorganisms11030793. Microorganisms. 2023. PMID: 36985366 Free PMC article. Review.
References
-
- Alexa Oniciuc E. A., Walsh C. J., Coughlan L. M., Awad A., Simon C. A., Ruiz L., Crispie F.. (2020) ‘Dairy Products and Dairy-Processing Environments as a Reservoir of Antibiotic Resistance and Quorum-Quenching Determinants as Revealed through Functional Metagenomics mSystems, 5, e00723–19. doi:10.1128/mSystems.00723-19, PMID: . - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

