Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling
- PMID: 36500666
- PMCID: PMC9739628
- DOI: 10.3390/molecules27238568
Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling
Abstract
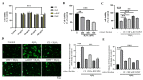
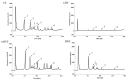
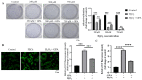
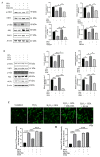
Eucommia ulmoides Oliver staminate flower (ESF) tea enjoys a good reputation in folk medicine and displays multiple bioactivities, such as antioxidant and antifatigue properties. However, the underlying biological mechanisms remain largely unknown. In this study, we aimed to investigate whether ESF tea can mitigate cellular oxidative stress. Crude ethyl alcohol extract and its three subfractions prepared by sequential extraction with chloroform, n-butyl alcohol and residual water were prepared from ESF tea. The results of antioxidant activity tests in vitro manifested n-butyl alcohol fraction (n-BUF) showed the strongest antioxidant capacity (DPPH: IC50 = 24.45 ± 0.74 μg/mL, ABTS: IC50 = 17.25 ± 0.04 μg/mL). Moreover, all subfractions of ESF tea, especially the n-BUF, exhibited an obvious capacity to scavenge the reactive oxygen species (ROS) and stimulate the NRF2 antioxidative response in human keratinocytes HaCaT treated by H2O2. Using ultra-high-performance liquid chromatography, we identified geniposidic acid (GPA) as the most abundant component in ESF tea extract. Furthermore, it was found that GPA relieved oxidative stress in H2O2-induced HaCaT cells by activating the Akt/Nrf2/OGG1 pathway. Our findings indicated that ESF tea may be a source of natural antioxidants to protect against skin cell oxidative damage and deserves further development and utilization.
Keywords: AKT; Eucommia ulmoides Oliver staminate flower tea; NRF2; geniposidic acid; keratinocyte; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Lignans from Eucommia ulmoides Oliver leaves exhibit neuroprotective effects via activation of the PI3K/Akt/GSK-3β/Nrf2 signaling pathways in H2O2-treated PC-12 cells.Phytomedicine. 2022 Jul;101:154124. doi: 10.1016/j.phymed.2022.154124. Epub 2022 Apr 19. Phytomedicine. 2022. PMID: 35487038
-
Eucommia ulmoides Oliv.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine.J Ethnopharmacol. 2014;151(1):78-92. doi: 10.1016/j.jep.2013.11.023. Epub 2013 Dec 1. J Ethnopharmacol. 2014. PMID: 24296089 Review.
-
Formation and stability of Eucommia ulmoides Oliver seed oil-loaded inverse microemulsion formed by food-grade ingredients and its antioxidant activities.J Food Sci. 2020 May;85(5):1489-1499. doi: 10.1111/1750-3841.15103. Epub 2020 Apr 13. J Food Sci. 2020. PMID: 32282076
-
Iridoid constituents from the male flower of Eucommia ulmoides and their promotion proliferation on ESF-1.J Asian Nat Prod Res. 2015;17(9):867-75. doi: 10.1080/10286020.2015.1039999. Epub 2015 May 21. J Asian Nat Prod Res. 2015. PMID: 25996193
-
Eucommia ulmoides Oliver: A Potential Feedstock for Bioactive Products.J Agric Food Chem. 2018 Jun 6;66(22):5433-5438. doi: 10.1021/acs.jafc.8b01312. Epub 2018 May 23. J Agric Food Chem. 2018. PMID: 29745662 Review.
Cited by
-
Investigating the Phytochemical Composition, Antioxidant, and Anti-Inflammatory Potentials of Cassinopsis ilicifolia (Hochst.) Kuntze Extract against Some Oxidative Stress and Inflammation Molecular Markers.Curr Issues Mol Biol. 2024 Sep 1;46(9):9639-9658. doi: 10.3390/cimb46090573. Curr Issues Mol Biol. 2024. PMID: 39329925 Free PMC article.
-
Mas receptor activation facilitates innate hematoma resolution and neurological recovery after hemorrhagic stroke in mice.J Neuroinflammation. 2024 Apr 24;21(1):106. doi: 10.1186/s12974-024-03105-8. J Neuroinflammation. 2024. PMID: 38658922 Free PMC article.
-
Biological properties and potential application of extracts and compounds from different medicinal parts (bark, leaf, staminate flower, and seed) of Eucommia ulmoides: A review.Heliyon. 2024 Mar 18;10(6):e27870. doi: 10.1016/j.heliyon.2024.e27870. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38545153 Free PMC article. Review.
References
-
- Zhang R.Q., Wang Y., Yang Y.Y. Research Progress in Study of Sheng Ji Zong Lu in Last Ten Years. Chin. J. Basic Med. Tradit. Chin. Med. 2022;28:486–490. doi: 10.19945/j.cnki.issn.1006-3250.2022.03.007. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

