Exploring the cardiac ECM during fibrosis: A new era with next-gen proteomics
- PMID: 36483540
- PMCID: PMC9722982
- DOI: 10.3389/fmolb.2022.1030226
Exploring the cardiac ECM during fibrosis: A new era with next-gen proteomics
Abstract
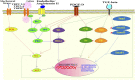
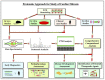
Extracellular matrix (ECM) plays a critical role in maintaining elasticity in cardiac tissues. Elasticity is required in the heart for properly pumping blood to the whole body. Dysregulated ECM remodeling causes fibrosis in the cardiac tissues. Cardiac fibrosis leads to stiffness in the heart tissues, resulting in heart failure. During cardiac fibrosis, ECM proteins get excessively deposited in the cardiac tissues. In the ECM, cardiac fibroblast proliferates into myofibroblast upon various kinds of stimulations. Fibroblast activation (myofibroblast) contributes majorly toward cardiac fibrosis. Other than cardiac fibroblasts, cardiomyocytes, epithelial/endothelial cells, and immune system cells can also contribute to cardiac fibrosis. Alteration in the expression of the ECM core and ECM-modifier proteins causes different types of cardiac fibrosis. These different components of ECM culminated into different pathways inducing transdifferentiation of cardiac fibroblast into myofibroblast. In this review, we summarize the role of different ECM components during cardiac fibrosis progression leading to heart failure. Furthermore, we highlight the importance of applying mass-spectrometry-based proteomics to understand the key changes occurring in the ECM during fibrotic progression. Next-gen proteomics studies will broaden the potential to identify key targets to combat cardiac fibrosis in order to achieve precise medicine-development in the future.
Keywords: cardiac fibrosis; extracellular matrix; heart failure; mass-spectrometry; proteomics.
Copyright © 2022 Sarohi, Chakraborty and Basak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Fibroblast growth factor-2 regulates human cardiac myofibroblast-mediated extracellular matrix remodeling.J Transl Med. 2015 May 7;13:147. doi: 10.1186/s12967-015-0510-4. J Transl Med. 2015. PMID: 25948488 Free PMC article.
-
The Extracellular Matrix in Ischemic and Nonischemic Heart Failure.Circ Res. 2019 Jun 21;125(1):117-146. doi: 10.1161/CIRCRESAHA.119.311148. Epub 2019 Jun 20. Circ Res. 2019. PMID: 31219741 Free PMC article. Review.
-
Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target.Front Cardiovasc Med. 2022 May 6;9:886553. doi: 10.3389/fcvm.2022.886553. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35600469 Free PMC article. Review.
-
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article.
-
Syndecans in heart fibrosis.Cell Tissue Res. 2016 Sep;365(3):539-52. doi: 10.1007/s00441-016-2454-2. Epub 2016 Jul 14. Cell Tissue Res. 2016. PMID: 27411689 Review.
Cited by
-
Preventive and treatment efficiency of dendrosomal nano-curcumin against ISO-induced cardiac fibrosis in mouse model.PLoS One. 2024 Oct 10;19(10):e0311817. doi: 10.1371/journal.pone.0311817. eCollection 2024. PLoS One. 2024. PMID: 39388499 Free PMC article.
-
Genetics and Molecular Basis of Congenital Heart Defects in Down Syndrome: Role of Extracellular Matrix Regulation.Int J Mol Sci. 2023 Feb 2;24(3):2918. doi: 10.3390/ijms24032918. Int J Mol Sci. 2023. PMID: 36769235 Free PMC article. Review.
-
FBXL8 inhibits post-myocardial infarction cardiac fibrosis by targeting Snail1 for ubiquitin-proteasome degradation.Cell Death Dis. 2024 Apr 13;15(4):263. doi: 10.1038/s41419-024-06646-1. Cell Death Dis. 2024. PMID: 38615011 Free PMC article.
-
Rodent Models of Dilated Cardiomyopathy and Heart Failure for Translational Investigations and Therapeutic Discovery.Int J Mol Sci. 2023 Feb 5;24(4):3162. doi: 10.3390/ijms24043162. Int J Mol Sci. 2023. PMID: 36834573 Free PMC article. Review.
-
Survivin regulates intracellular stiffness and extracellular matrix production in vascular smooth muscle cells.APL Bioeng. 2023 Oct 20;7(4):046104. doi: 10.1063/5.0157549. eCollection 2023 Dec. APL Bioeng. 2023. PMID: 37868708 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

