Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism
- PMID: 36483153
- PMCID: PMC9724488
- DOI: 10.3748/wjg.v28.i43.6131
Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism
Abstract
Background: Studies have shown that a high-fat diet (HFD) can alter gut microbiota (GM) homeostasis and participate in lipid metabolism disorders associated with obesity. Therefore, regulating the construction of GM with the balance of lipid metabolism has become essential for treating obesity. Salvia miltiorrhiza extract (Sal), a common traditional Chinese medicine, has been proven effective against atherosclerosis, hyperlipidemia, obesity, and other dyslipidemia-related diseases.
Aim: To investigate the anti-obesity effects of Sal in rats with HFD-induced obesity, and explore the underlying mechanism by focusing on GM and lipid metabolism.
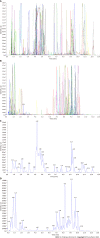
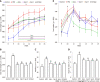
Methods: Obesity was induced in rats with an HFD for 7 wk, and Sal (0.675 g/1.35 g/2.70 g/kg/d) was administered to treat obese rats for 8 wk. The therapeutic effect was evaluated by body weight, body fat index, waistline, and serum lipid level. Lipid factors (cAMP, PKA, and HSL) in liver and fat homogenates were analyzed by ELISA. The effect of Sal on GM and lipid metabolism was assessed by 16S rRNA-based microbiota analysis and untargeted lipidomic analysis (LC-MS/MS), respectively.
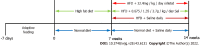
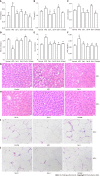
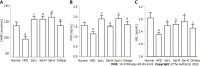
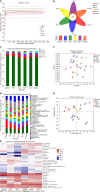
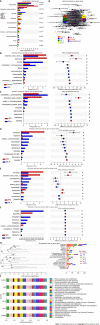
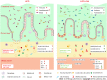
Results: Sal treatment markedly reduced weight, body fat index, serum triglycerides (TG), total cholesterol (TC), low-density lipoprotein, glucose, free fatty acid, hepatic lipid accumulation, and adipocyte vacuolation, and increased serum high-density lipoprotein (HDL-C) in rats with HFD-induced obesity. These effects were associated with increased concentrations of lipid factors such as cAMP, PKA, and HSL in the liver and adipose tissues, enhanced gut integrity, and improved lipid metabolism. GM analysis revealed that Sal could reverse HFD-induced dysbacteriosis by promoting the abundance of Actinobacteriota and Proteobacteria, and decreasing the growth of Firmicutes and Desulfobacterita. Furthermore, LC-MS/MS analysis indicated that Sal decreased TGs (TG18:2/18:2/20:4, TG16:0/18:2/22:6), DGs (DG14:0/22:6, DG22:6/22:6), CL (18:2/ 18:1/18:1/20:0), and increased ceramides (Cers; Cer d16:0/21:0, Cer d16:1/24:1), (O-acyl)-ω-hydroxy fatty acids (OAHFAs; OAHFA18:0/14:0) in the feces of rats. Spearman's correlation analysis further indicated that TGs, DGs, and CL were negatively related to the abundance of Facklamia and Dubosiella, and positively correlated with Blautia and Quinella, while OAHFAs and Cers were the opposite.
Conclusion: Sal has an anti-obesity effect by regulating the GM and lipid metabolism.
Keywords: Gut microbiota; High fat diet; Lipid metabolism; Obesity; Salvia miltiorrhiza extract.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There are no conflicts of interest to report.
Figures

Similar articles
-
Aqueous extract of fermented Eucommia ulmoides leaves alleviates hyperlipidemia by maintaining gut homeostasis and modulating metabolism in high-fat diet fed rats.Phytomedicine. 2024 Jun;128:155291. doi: 10.1016/j.phymed.2023.155291. Epub 2023 Dec 27. Phytomedicine. 2024. PMID: 38518640
-
AMPK-Mediated Hypolipidemic Effects of a Salvia miltiorrhiza and Paeonia lactiflora Mixed Extract on High-Fat Diet-Induced Liver Triglyceride Accumulation: An In Vivo and In Vitro Study.Nutrients. 2024 Sep 20;16(18):3189. doi: 10.3390/nu16183189. Nutrients. 2024. PMID: 39339790 Free PMC article.
-
Eucommia bark/leaf extract improves HFD-induced lipid metabolism disorders via targeting gut microbiota to activate the Fiaf-LPL gut-liver axis and SCFAs-GPR43 gut-fat axis.Phytomedicine. 2023 Feb;110:154652. doi: 10.1016/j.phymed.2023.154652. Epub 2023 Jan 6. Phytomedicine. 2023. PMID: 36638713
-
Role of herbal medicine and gut microbiota in the prevention and treatment of obesity.J Ethnopharmacol. 2023 Apr 6;305:116127. doi: 10.1016/j.jep.2022.116127. Epub 2023 Jan 2. J Ethnopharmacol. 2023. PMID: 36603782 Review.
-
Protective effect of hydroxychloroquine on rheumatoid arthritis-associated atherosclerosis.Animal Model Exp Med. 2019 Apr 19;2(2):98-106. doi: 10.1002/ame2.12065. eCollection 2019 Jun. Animal Model Exp Med. 2019. PMID: 31392302 Free PMC article. Review.
Cited by
-
Traditional Chinese medicine to improve immune imbalance of asthma: focus on the adjustment of gut microbiota.Front Microbiol. 2024 Oct 1;15:1409128. doi: 10.3389/fmicb.2024.1409128. eCollection 2024. Front Microbiol. 2024. PMID: 39411430 Free PMC article. Review.
-
Recent advances of traditional Chinese medicine against cardiovascular disease: overview and potential mechanisms.Front Endocrinol (Lausanne). 2024 Sep 30;15:1366285. doi: 10.3389/fendo.2024.1366285. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39403576 Free PMC article. Review.
-
Salvia miltiorrhiza bge. f. alba ameliorates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease via the STING pathway.Am J Transl Res. 2024 Aug 15;16(8):3678-3689. doi: 10.62347/XUNO9933. eCollection 2024. Am J Transl Res. 2024. PMID: 39262750 Free PMC article.
-
Matcha alleviates obesity by modulating gut microbiota and its metabolites.Curr Res Food Sci. 2024 Aug 17;9:100823. doi: 10.1016/j.crfs.2024.100823. eCollection 2024. Curr Res Food Sci. 2024. PMID: 39253721 Free PMC article.
-
Konjac supplementation can alleviate obesity induced by high-fat diet in mice by modulating gut microbiota and its metabolites.Curr Res Food Sci. 2024 Jul 14;9:100805. doi: 10.1016/j.crfs.2024.100805. eCollection 2024. Curr Res Food Sci. 2024. PMID: 39131951 Free PMC article.
References
-
- Acín-Pérez R, Iborra S, Martí-Mateos Y, Cook ECL, Conde-Garrosa R, Petcherski A, Muñoz MDM, Martínez de Mena R, Krishnan KC, Jiménez C, Bolaños JP, Laakso M, Lusis AJ, Shirihai OS, Sancho D, Enríquez JA. Fgr kinase is required for proinflammatory macrophage activation during diet-induced obesity. Nat Metab. 2020;2:974–988. - PMC - PubMed
-
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. - PubMed
-
- Zigmond E, Zangen SW, Pappo O, Sklair-Levy M, Lalazar G, Zolotaryova L, Raz I, Ilan Y. Beta-glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. Am J Physiol Endocrinol Metab. 2009;296:E72–E78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

