Aporphine and isoquinoline derivatives block glioblastoma cell stemness and enhance temozolomide cytotoxicity
- PMID: 36477472
- PMCID: PMC9729571
- DOI: 10.1038/s41598-022-25534-2
Aporphine and isoquinoline derivatives block glioblastoma cell stemness and enhance temozolomide cytotoxicity
Abstract
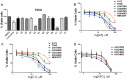
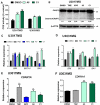
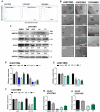
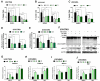
Glioblastoma (GBM) is the most aggressive and common primary malignant brain tumor with limited available therapeutic approaches. Despite improvements in therapeutic options for GBM patients, efforts to develop new successful strategies remain as major unmet medical needs. Based on the cytotoxic properties of aporphine compounds, we evaluated the biological effect of 12 compounds obtained through total synthesis of ( ±)-apomorphine hydrochloride (APO) against GBM cells. The compounds 2,2,2-trifluoro-1-(1-methylene-3,4-dihydroisoquinolin-2(1H)-yl)ethenone (A5) and ( ±)-1-(10,11-dimethoxy-6a,7-dihydro-4H-dibenzo[de,g]quinolin-6(5H)-yl)ethenone (C1) reduced the viability of GBM cells, with 50% inhibitory concentration ranging from 18 to 48 μM in patient-derived GBM cultures. Our data show that APO, A5 or C1 modulate the expression of DNA damage and apoptotic markers, impair 3D-gliomasphere growth and reduce the expression of stemness markers. Potential activity and protein targets of A5, C1 or APO were predicted in silico based on PASS and SEA software. Dopamine receptors (DRD1 and 5), CYP2B6, CYP2C9 and ABCB1, whose transcripts were differentially expressed in the GBM cells, were among the potential A5 or C1 target proteins. Docking analyses (HQSAR and 3D-QSAR) were performed to characterize possible interactions of ABCB1 and CYP2C9 with the compounds. Notably, A5 or C1 treatment, but not temozolomide (TMZ), reduced significantly the levels of extracellular ATP, suggesting ABCB1 negative regulation, which was correlated with stronger cytotoxicity induced by the combination of TMZ with A5 or C1 on GBM cells. Hence, our data reveal a potential therapeutic application of A5 and C1 as cytotoxic agents against GBM cells and predicted molecular networks that can be further exploited to characterize the pharmacological effects of these isoquinoline-containing substances.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Combined treatment with 2'-hydroxycinnamaldehyde and temozolomide suppresses glioblastoma tumorspheres by decreasing stemness and invasiveness.J Neurooncol. 2019 May;143(1):69-77. doi: 10.1007/s11060-019-03151-w. Epub 2019 Mar 18. J Neurooncol. 2019. PMID: 30887242
-
Xanthohumol regulates miR-4749-5p-inhibited RFC2 signaling in enhancing temozolomide cytotoxicity to glioblastoma.Life Sci. 2020 Aug 1;254:117807. doi: 10.1016/j.lfs.2020.117807. Epub 2020 May 16. Life Sci. 2020. PMID: 32422304
-
The protein kinase LKB1 promotes self-renewal and blocks invasiveness in glioblastoma.J Cell Physiol. 2022 Jan;237(1):743-762. doi: 10.1002/jcp.30542. Epub 2021 Aug 5. J Cell Physiol. 2022. PMID: 34350982
-
Discovery of Aporphine Analogues as Potential Antiplatelet and Antioxidant Agents: Design, Synthesis, Structure-Activity Relationships, Biological Evaluations, and in silico Molecular Docking Studies.ChemMedChem. 2018 Sep 6;13(17):1817-1832. doi: 10.1002/cmdc.201800318. Epub 2018 Aug 8. ChemMedChem. 2018. PMID: 30088331
-
Involvement of Intracellular Cholesterol in Temozolomide-Induced Glioblastoma Cell Death.Neurol Med Chir (Tokyo). 2018 Jul 15;58(7):296-302. doi: 10.2176/nmc.ra.2018-0040. Epub 2018 Jun 13. Neurol Med Chir (Tokyo). 2018. PMID: 29899179 Free PMC article. Review.
Cited by
-
An Optimized Liquid Chromatography-Mass Spectrometry Method for Ganglioside Analysis in Cell Lines.Cells. 2024 Oct 2;13(19):1640. doi: 10.3390/cells13191640. Cells. 2024. PMID: 39404403 Free PMC article.
-
Lysicamine Reduces Protein Kinase B (AKT) Activation and Promotes Necrosis in Anaplastic Thyroid Cancer.Pharmaceuticals (Basel). 2023 Dec 4;16(12):1687. doi: 10.3390/ph16121687. Pharmaceuticals (Basel). 2023. PMID: 38139812 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

