The insulin and IGF signaling pathway sustains breast cancer stem cells by IRS2/PI3K-mediated regulation of MYC
- PMID: 36476848
- PMCID: PMC9793643
- DOI: 10.1016/j.celrep.2022.111759
The insulin and IGF signaling pathway sustains breast cancer stem cells by IRS2/PI3K-mediated regulation of MYC
Abstract
Despite the strong association of the insulin/insulin-like growth factor (IGF) signaling (IIS) pathway with tumor initiation, recurrence, and metastasis, the mechanism by which this pathway regulates cancer progression is not well understood. Here, we report that IIS supports breast cancer stem cell (CSC) self-renewal in an IRS2-phosphatidylinositol 3-kinase (PI3K)-dependent manner that involves the activation and stabilization of MYC. IRS2-PI3K signaling enhances MYC expression through the inhibition of GSK3β activity and suppression of MYC phosphorylation on threonine 58, thus reducing proteasome-mediated degradation of MYC and sustaining active pS62-MYC function. A stable T58A-Myc mutant rescues CSC function in Irs2-/- cells, supporting the role of this MYC stabilization in IRS2-dependent CSC regulation. These findings establish a mechanistic connection between the IIS pathway and MYC and highlight a role for IRS2-dependent signaling in breast cancer progression.
Keywords: CP: Cancer; CSC; IGF; IRS2; MYC; PI3K; breast cancer; cancer stem cell; insulin; insulin receptor substrate; insulin-like growth factor.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
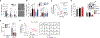
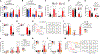

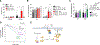
Similar articles
-
Identification of a Novel Invasion-Promoting Region in Insulin Receptor Substrate 2.Mol Cell Biol. 2018 Jun 28;38(14):e00590-17. doi: 10.1128/MCB.00590-17. Print 2018 Jul 15. Mol Cell Biol. 2018. PMID: 29685905 Free PMC article.
-
Irs2 inactivation suppresses tumor progression in Pten+/- mice.Am J Pathol. 2009 Jan;174(1):276-86. doi: 10.2353/ajpath.2009.080086. Epub 2008 Dec 18. Am J Pathol. 2009. PMID: 19095950 Free PMC article.
-
The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors.Breast Cancer Res. 2013 May 12;15(3):R39. doi: 10.1186/bcr3423. Breast Cancer Res. 2013. PMID: 23663564 Free PMC article.
-
Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2.Can J Physiol Pharmacol. 2014 Aug;92(8):613-20. doi: 10.1139/cjpp-2014-0114. Epub 2014 Jun 3. Can J Physiol Pharmacol. 2014. PMID: 24977713 Review.
-
PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in IGF-1-induced VEGF-C upregulation in breast cancer.J Cancer Res Clin Oncol. 2011 Nov;137(11):1587-94. doi: 10.1007/s00432-011-1049-2. Epub 2011 Sep 9. J Cancer Res Clin Oncol. 2011. PMID: 21904903 Review.
Cited by
-
ULK2 suppresses ovarian cancer cell migration and invasion by elevating IGFBP3.PeerJ. 2024 Jun 28;12:e17628. doi: 10.7717/peerj.17628. eCollection 2024. PeerJ. 2024. PMID: 38952983 Free PMC article.
-
Pharmacological impact of microRNAs in head and neck squamous cell carcinoma: Prevailing insights on molecular pathways, diagnosis, and nanomedicine treatment.Front Pharmacol. 2023 May 3;14:1174330. doi: 10.3389/fphar.2023.1174330. eCollection 2023. Front Pharmacol. 2023. PMID: 37205904 Free PMC article. Review.
-
Fluorescent tagging of endogenous IRS2 with an auxin-dependent degron to assess dynamic intracellular localization and function.J Biol Chem. 2024 Nov;300(11):107796. doi: 10.1016/j.jbc.2024.107796. Epub 2024 Sep 19. J Biol Chem. 2024. PMID: 39305958 Free PMC article.
-
Introducing effective genes in lymph node metastasis of breast cancer patients using SHAP values based on the mRNA expression data.PLoS One. 2024 Aug 16;19(8):e0308531. doi: 10.1371/journal.pone.0308531. eCollection 2024. PLoS One. 2024. PMID: 39150915 Free PMC article.
-
Ginseng-derived compounds as potential anticancer agents targeting cancer stem cells.J Ginseng Res. 2024 May;48(3):266-275. doi: 10.1016/j.jgr.2024.03.003. Epub 2024 Mar 12. J Ginseng Res. 2024. PMID: 38707642 Free PMC article. Review.
References
-
- Chaffer CL, and Weinberg RA (2011). A perspective on cancer cell metastasis. science 331, 1559–1564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

