Antibodies to watch in 2023
- PMID: 36472472
- PMCID: PMC9728470
- DOI: 10.1080/19420862.2022.2153410
Antibodies to watch in 2023
Abstract
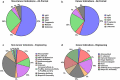
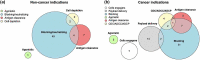
In this 14th installment of the annual Antibodies to Watch article series, we discuss key events in commercial monoclonal antibody therapeutics development that occurred in 2022 and forecast events that might occur in 2023. As of mid-November, 12 antibody therapeutics had been granted first approvals in either the United States or European Union (tebentafusp (Kimmtrak), faricimab (Vabysmo), sutimlimab (Enjaymo), relatlimab (Opdualag), tixagevimab/cilgavimab (Evusheld), mosunetuzumab (Lunsumio), teclistamab (TECVAYLI), spesolimab (SPEVIGO), tremelimumab (Imjudo; combo with durvalumab), nirsevimab (Beyfortus), mirvetuximab soravtansine (ELAHERE™), and teplizumab (TZIELD)), including 4 bispecific antibodies and 1 ADC. Based on FDA action dates, several additional product candidates could be approved by the end of 2022. An additional seven were first approved in China or Japan in 2022, including two bispecific antibodies (cadonilimab and ozoralizumab). Globally, at least 24 investigational antibody therapeutics are undergoing review by regulatory agencies as of mid-November 2022. Our data show that, with antibodies for COVID-19 excluded, the late-stage commercial clinical pipeline grew by ~20% in the past year to include nearly 140 investigational antibody therapeutics that were designed using a wide variety of formats and engineering techniques. Of those in late-stage development, marketing application submissions for at least 23 may occur by the end of 2023, of which 5 are bispecific (odronextamab, erfonrilimab, linvoseltamab, zanidatamab, and talquetamab) and 2 are ADCs (datopotamab deruxtecan, and tusamitamab ravtansine).
Keywords: Antibody therapeutics; COVID-19; SARS-CoV-2; cancer; european medicines agency; food and drug administration; immune-mediated disorders.
Conflict of interest statement
HK and JV are employed by companies that develop antibody therapeutics. JMR is employed by The Antibody Society, a non-profit trade association funded by corporate sponsors that develop antibody therapeutics or provide services to companies that develop antibody therapeutics, and she is Editor-in-Chief of
Figures

Similar articles
-
Antibodies to watch in 2022.MAbs. 2022 Jan-Dec;14(1):2014296. doi: 10.1080/19420862.2021.2014296. MAbs. 2022. PMID: 35030985 Free PMC article. Review.
-
Antibodies to watch in 2024.MAbs. 2024 Jan-Dec;16(1):2297450. doi: 10.1080/19420862.2023.2297450. Epub 2024 Jan 5. MAbs. 2024. PMID: 38178784 Free PMC article.
-
Antibodies to watch in 2021.MAbs. 2021 Jan-Dec;13(1):1860476. doi: 10.1080/19420862.2020.1860476. MAbs. 2021. PMID: 33459118 Free PMC article. Review.
-
Antibodies to watch in 2019.MAbs. 2019 Feb/Mar;11(2):219-238. doi: 10.1080/19420862.2018.1556465. Epub 2018 Dec 22. MAbs. 2019. PMID: 30516432 Free PMC article.
-
Antibodies to watch in 2018.MAbs. 2018 Feb/Mar;10(2):183-203. doi: 10.1080/19420862.2018.1415671. Epub 2018 Jan 16. MAbs. 2018. PMID: 29300693 Free PMC article.
Cited by
-
A comprehensive overview of psoriatic research over the past 20 years: machine learning-based bibliometric analysis.Front Immunol. 2023 Oct 26;14:1272080. doi: 10.3389/fimmu.2023.1272080. eCollection 2023. Front Immunol. 2023. PMID: 37954610 Free PMC article.
-
Modular Nanotransporters Delivering Biologically Active Molecules to the Surface of Mitochondria.Pharmaceutics. 2023 Nov 27;15(12):2687. doi: 10.3390/pharmaceutics15122687. Pharmaceutics. 2023. PMID: 38140028 Free PMC article.
-
Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies?Mar Drugs. 2023 Sep 16;21(9):496. doi: 10.3390/md21090496. Mar Drugs. 2023. PMID: 37755109 Free PMC article. Review.
-
Unlocking the Power of Complement-Dependent Cytotoxicity: Engineering Strategies for the Development of Potent Therapeutic Antibodies for Cancer Treatments.BioDrugs. 2023 Sep;37(5):637-648. doi: 10.1007/s40259-023-00618-1. Epub 2023 Jul 24. BioDrugs. 2023. PMID: 37486566 Review.
-
Druggability properties of a L309K mutation in the antibody CH2 domain.3 Biotech. 2024 Jun;14(6):152. doi: 10.1007/s13205-024-04000-y. Epub 2024 May 11. 3 Biotech. 2024. PMID: 38742229
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
