Enhanced tumor penetration for efficient chemotherapy by a magnetothermally sensitive micelle combined with magnetic targeting and magnetic hyperthermia
- PMID: 36467035
- PMCID: PMC9715748
- DOI: 10.3389/fphar.2022.1045976
Enhanced tumor penetration for efficient chemotherapy by a magnetothermally sensitive micelle combined with magnetic targeting and magnetic hyperthermia
Abstract
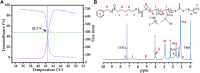
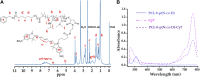
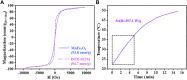
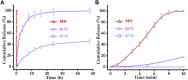
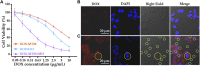
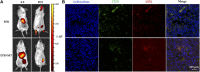
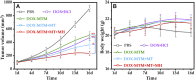
The high accumulation and poor penetration of nanocarriers in tumor is a contradiction of nanomedicine, which reduces the efficacy of chemotherapy. Due to the positive effect of hyperthermia on in vivo drug diffusion, we designed a magnetothermally sensitive micelle (MTM) by integrating magnetic targeting (MT), magnetic hyperthermia (MH), and magnetothermally responsive drug release to facilitate simultaneous drug accumulation and penetration in tumor. Accordingly, we synthesized a cyanine7-modified thermosensitive polymer with phase transition at 42.3°C, and utilized it to prepare drug-loaded MTMs by encapsulating superparamagnetic MnFe2O4 nanoparticles and doxorubicin (DOX). The obtained DOX-MTM had not only high contents of DOX (9.1%) and MnFe2O4 (38.7%), but also some advantages such as superparamagnetism, high saturation magnetization, excellent magnetocaloric effect, and magnetothermal-dependent drug release. Therefore, DOX-MTM improved in vitro DOX cytotoxicity by enhancing DOX endocytosis under the assistance of MH. Furthermore, MT and MH enhanced in vivo DOX-MTM accumulation and DOX penetration in tumor, respectively, substantially inhibiting tumor growth (84%) with excellent biosafety. These results indicate the development of an optimized drug delivery system with MH and MH-dependent drug release, introducing a feasible strategy to enhance the application of nanomedicines in tumor chemotherapy.
Keywords: enhanced tumor penetration; magnetic hyperthermia; magnetic targeting; magnetothermal-responsive drug release; thermosensitive polymer.
Copyright © 2022 Wang, Wang, Chen, Chen, Zheng, Xin, Zhou, Song and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Enhanced Synergism of Thermo-chemotherapy For Liver Cancer with Magnetothermally Responsive Nanocarriers.Theranostics. 2018 Jan 1;8(3):693-709. doi: 10.7150/thno.21297. eCollection 2018. Theranostics. 2018. PMID: 29344299 Free PMC article.
-
Magnetocaloric effect in magnetothermally-responsive nanocarriers for hyperthermia-triggered drug release.Nanotechnology. 2012 Dec 21;23(50):505706. doi: 10.1088/0957-4484/23/50/505706. Epub 2012 Nov 26. Nanotechnology. 2012. PMID: 23183247
-
Preparation and Evaluation of Doxorubicin-Loaded PLA-PEG-FA Copolymer Containing Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Cancer Treatment: Combination Therapy with Hyperthermia and Chemotherapy.Int J Nanomedicine. 2020 Aug 18;15:6167-6182. doi: 10.2147/IJN.S261638. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32922000 Free PMC article.
-
Actively Targeted Magnetothermally Responsive Nanocarriers/Doxorubicin for Thermochemotherapy of Hepatoma.ACS Appl Mater Interfaces. 2018 Dec 5;10(48):41107-41117. doi: 10.1021/acsami.8b14972. Epub 2018 Nov 19. ACS Appl Mater Interfaces. 2018. PMID: 30403475
-
A Theranostic Nanocomplex Combining with Magnetic Hyperthermia for Enhanced Accumulation and Efficacy of pH-Triggering Polymeric Cisplatin(IV) Prodrugs.Pharmaceuticals (Basel). 2022 Apr 14;15(4):480. doi: 10.3390/ph15040480. Pharmaceuticals (Basel). 2022. PMID: 35455477 Free PMC article.
Cited by
-
Smart delivery vehicles for cancer: categories, unique roles and therapeutic strategies.Nanoscale Adv. 2024 Jun 20;6(17):4275-4308. doi: 10.1039/d4na00285g. eCollection 2024 Aug 20. Nanoscale Adv. 2024. PMID: 39170969 Free PMC article. Review.
-
Drug-Loaded Lipid Magnetic Nanoparticles for Combined Local Hyperthermia and Chemotherapy against Glioblastoma Multiforme.ACS Nano. 2023 Sep 26;17(18):18441-18455. doi: 10.1021/acsnano.3c06085. Epub 2023 Sep 12. ACS Nano. 2023. PMID: 37698887 Free PMC article.
References
-
- Aguiar J., Carpena P., Molina-Bolívar J. A., Carnero Ruiz C. (2003). On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 258, 116–122. 10.1016/S0021-9797(02)00082-6 - DOI
-
- Bian Q., Yan X., Lang M. (2012). Thermoresponsive biotinylated star amphiphilic block copolymer: Synthesis, self-assembly, and specific target recognition. Polymer 53, 1684–1693. 10.1016/j.polymer.2012.02.031 - DOI
LinkOut - more resources
Full Text Sources

