What role for cellular metabolism in the control of hepatitis viruses?
- PMID: 36466918
- PMCID: PMC9713817
- DOI: 10.3389/fimmu.2022.1033314
What role for cellular metabolism in the control of hepatitis viruses?
Abstract
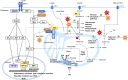
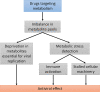
Hepatitis B, C and D viruses (HBV, HCV, HDV, respectively) specifically infect human hepatocytes and often establish chronic viral infections of the liver, thus escaping antiviral immunity for years. Like other viruses, hepatitis viruses rely on the cellular machinery to meet their energy and metabolite requirements for replication. Although this was initially considered passive parasitism, studies have shown that hepatitis viruses actively rewire cellular metabolism through molecular interactions with specific enzymes such as glucokinase, the first rate-limiting enzyme of glycolysis. As part of research efforts in the field of immunometabolism, it has also been shown that metabolic changes induced by viruses could have a direct impact on the innate antiviral response. Conversely, detection of viral components by innate immunity receptors not only triggers the activation of the antiviral defense but also induces in-depth metabolic reprogramming that is essential to support immunological functions. Altogether, these complex triangular interactions between viral components, innate immunity and hepatocyte metabolism may explain why chronic hepatitis infections progressively lead to liver inflammation and progression to cirrhosis, fibrosis and hepatocellular carcinoma (HCC). In this manuscript, we first present a global overview of known connections between the innate antiviral response and cellular metabolism. We then report known molecular mechanisms by which hepatitis viruses interfere with cellular metabolism in hepatocytes and discuss potential consequences on the innate immune response. Finally, we present evidence that drugs targeting hepatocyte metabolism could be used as an innovative strategy not only to deprive viruses of key metabolites, but also to restore the innate antiviral response that is necessary to clear infection.
Keywords: cellular metabolism; hepatitis virus; hepatocyte; immunometabolism; inflammation; innate immunity; liver diseases.
Copyright © 2022 Diaz, Vidalain, Ramière, Lotteau and Perrin-Cocon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[Role of cellular metabolism in the control of chronic viral hepatitis].Med Sci (Paris). 2023 Oct;39(10):754-762. doi: 10.1051/medsci/2023125. Epub 2023 Nov 9. Med Sci (Paris). 2023. PMID: 37943136 French.
-
Innate immune recognition and modulation in hepatitis D virus infection.World J Gastroenterol. 2020 Jun 7;26(21):2781-2791. doi: 10.3748/wjg.v26.i21.2781. World J Gastroenterol. 2020. PMID: 32550754 Free PMC article. Review.
-
A new HDV mouse model identifies mitochondrial antiviral signaling protein (MAVS) as a key player in IFN-β induction.J Hepatol. 2017 Oct;67(4):669-679. doi: 10.1016/j.jhep.2017.05.010. Epub 2017 May 18. J Hepatol. 2017. PMID: 28527664
-
Hepatocellular carcinoma and infections with multiple hepatitis viruses.Princess Takamatsu Symp. 1995;25:61-6. Princess Takamatsu Symp. 1995. PMID: 8875610
-
Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma.World J Gastroenterol. 2020 Oct 14;26(38):5759-5783. doi: 10.3748/wjg.v26.i38.5759. World J Gastroenterol. 2020. PMID: 33132633 Free PMC article. Review.
Cited by
-
In vivo Hyperpolarized Metabolic Imaging to Monitor the Progression of Hepatitis B Virus (HBV)-Related Hepatitis to Liver Fibrosis.Mol Imaging Biol. 2024 Aug;26(4):649-657. doi: 10.1007/s11307-024-01936-8. Epub 2024 Jul 11. Mol Imaging Biol. 2024. PMID: 38992246
-
The Novel Link between Gene Expression Profiles of Adult T-Cell Leukemia/Lymphoma Patients' Peripheral Blood Lymphocytes and Ferroptosis Susceptibility.Genes (Basel). 2023 Oct 27;14(11):2005. doi: 10.3390/genes14112005. Genes (Basel). 2023. PMID: 38002949 Free PMC article.
-
Innate immunity and early liver inflammation.Front Immunol. 2023 May 2;14:1175147. doi: 10.3389/fimmu.2023.1175147. eCollection 2023. Front Immunol. 2023. PMID: 37205101 Free PMC article. Review.
-
Interactions of SARS-CoV-2 with Human Target Cells-A Metabolic View.Int J Mol Sci. 2024 Sep 16;25(18):9977. doi: 10.3390/ijms25189977. Int J Mol Sci. 2024. PMID: 39337465 Free PMC article. Review.
-
Understanding lactate in the development of Hepatitis B virus-related hepatocellular carcinoma.Infect Agent Cancer. 2024 Jul 15;19(1):31. doi: 10.1186/s13027-024-00593-4. Infect Agent Cancer. 2024. PMID: 39010155 Free PMC article. Review.
References
-
- Perrin-Cocon L, Diaz O, Aublin-Gex A, Vidalain P-O, Lotteau V. Reprogramming of central carbon metabolism in myeloid cells upon innate immune receptor stimulation. Immuno (2021) 1:1–14. doi: 10.3390/immuno1010001 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

