Diagnostic and therapeutic potential of RNASET2 in Crohn's disease: Disease-risk polymorphism modulates allelic-imbalance in expression and circulating protein levels and recombinant-RNASET2 attenuates pro-inflammatory cytokine secretion
- PMID: 36466822
- PMCID: PMC9709281
- DOI: 10.3389/fimmu.2022.999155
Diagnostic and therapeutic potential of RNASET2 in Crohn's disease: Disease-risk polymorphism modulates allelic-imbalance in expression and circulating protein levels and recombinant-RNASET2 attenuates pro-inflammatory cytokine secretion
Abstract
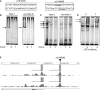
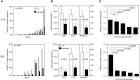
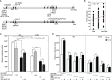
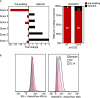
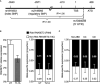
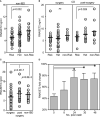
Ribonuclease T2 gene (RNASET2) variants are associated in genome wide association studies (GWAS) with risk for several autoimmune diseases, including Crohn's disease (CD). In T cells, a functional and biological relationship exists between TNFSF15-mediated enhancement of IFN-γ production, mucosal inflammation and RNASET2. Disease risk variants are associated with decreased mRNA expression and clinical characteristics of severe CD; however, functional classifications of variants and underlying molecular mechanisms contributing to pathogenesis remain largely unknown. In this study we demonstrate that allelic imbalance of RNASET2 disease risk variant rs2149092 is associated with transcriptional and post-transcriptional mechanisms regulating transcription factor binding, promoter-transactivation and allele-specific expression. RNASET2 mRNA expression decreases in response to multiple modes of T cell activation and recovers following elimination of activator. In CD patients with severe disease necessitating surgical intervention, preoperative circulating RNASET2 protein levels were decreased compared to non-IBD subjects and rebounded post-operatively following removal of the inflamed region, with levels associated with allelic carriage. Furthermore, overexpression or treatment with recombinant RNASET2 significantly reduced IFN-γ secretion. These findings reveal that RNASET2 cis- and trans-acting variation contributed regulatory complexity and determined expression and provide a basis for linking genetic variation with CD pathobiology. These data may ultimately identify RNASET2 as an effective therapeutic target in a subset of CD patients with severe disease.
Keywords: Crohn’s disease; allelic imbalance; cytokine; gene regulation; human; inflammation.
Copyright © 2022 Biener-Ramanujan, Rosier, Coetzee, McGovern, Hazelett, Targan and Gonsky.
Conflict of interest statement
ST – is a stockholder and a consultant for Prometheus Biosciences; DM - is a stockholder and a consultant for Prometheus Biosciences Cedars-Sinai - has financial interests in Prometheus Biosciences, Inc., a company which has access to the data and specimens in Cedars-Sinai’s MIRIAD Biobank (including the data and specimens used in this study) and seeks to develop commercial products. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Association of Ribonuclease T2 Gene Polymorphisms With Decreased Expression and Clinical Characteristics of Severity in Crohn's Disease.Gastroenterology. 2017 Jul;153(1):219-232. doi: 10.1053/j.gastro.2017.04.002. Epub 2017 Apr 9. Gastroenterology. 2017. PMID: 28400196 Free PMC article.
-
TNFSF15 transcripts from risk haplotype for Crohn's disease are overexpressed in stimulated T cells.Hum Mol Genet. 2009 Mar 15;18(6):1089-98. doi: 10.1093/hmg/ddp005. Epub 2009 Jan 5. Hum Mol Genet. 2009. PMID: 19124533
-
Genome-wide association study of Crohn's disease in Koreans revealed three new susceptibility loci and common attributes of genetic susceptibility across ethnic populations.Gut. 2014 Jan;63(1):80-7. doi: 10.1136/gutjnl-2013-305193. Epub 2013 Jul 14. Gut. 2014. PMID: 23850713
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Visceral adiposity and inflammatory bowel disease.Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review.
Cited by
-
Transcriptome-wide association studies associated with Crohn's disease: challenges and perspectives.Cell Biosci. 2024 Feb 25;14(1):29. doi: 10.1186/s13578-024-01204-w. Cell Biosci. 2024. PMID: 38403629 Free PMC article. Review.
-
A broken network of susceptibility genes in the monocytes of Crohn's disease patients.Life Sci Alliance. 2024 Jun 26;7(9):e202302394. doi: 10.26508/lsa.202302394. Print 2024 Sep. Life Sci Alliance. 2024. PMID: 38925865 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

