Utilization of macrocyclic peptides to target protein-protein interactions in cancer
- PMID: 36465350
- PMCID: PMC9714258
- DOI: 10.3389/fonc.2022.992171
Utilization of macrocyclic peptides to target protein-protein interactions in cancer
Abstract
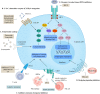
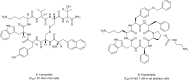
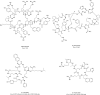
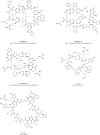
Protein-protein interactions (PPIs) play vital roles in normal cellular processes. Dysregulated PPIs are involved in the process of various diseases, including cancer. Thus, these PPIs may serve as potential therapeutic targets in cancer treatment. However, despite rapid advances in small-molecule drugs and biologics, it is still hard to target PPIs, especially for those intracellular PPIs. Macrocyclic peptides have gained growing attention for their therapeutic properties in targeting dysregulated PPIs. Macrocyclic peptides have some unique features, such as moderate sizes, high selectivity, and high binding affinities, which make them good drug candidates. In addition, some oncology macrocyclic peptide drugs have been approved by the US Food and Drug Administration (FDA) for clinical use. Here, we reviewed the recent development of macrocyclic peptides in cancer treatment. The opportunities and challenges were also discussed to inspire new perspectives.
Keywords: cancer; drug; macrocyclic peptide; protein-protein interactions; treatment.
Copyright © 2022 Yang, Zhu, Wu, Qu, Liu, Jiang, Ge and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
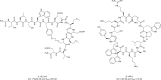

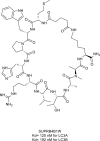
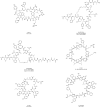

Similar articles
-
Cyclic and Macrocyclic Peptides as Chemical Tools To Recognise Protein Surfaces and Probe Protein-Protein Interactions.ChemMedChem. 2016 Apr 19;11(8):787-94. doi: 10.1002/cmdc.201500450. Epub 2015 Nov 13. ChemMedChem. 2016. PMID: 26563831 Free PMC article. Review.
-
Targeting protein-protein interfaces using macrocyclic peptides.Biopolymers. 2015 Jul;104(4):310-6. doi: 10.1002/bip.22625. Biopolymers. 2015. PMID: 25664609 Free PMC article. Review.
-
Targeting intracellular protein-protein interactions with macrocyclic peptides.Trends Pharmacol Sci. 2022 Mar;43(3):234-248. doi: 10.1016/j.tips.2021.11.008. Epub 2021 Dec 13. Trends Pharmacol Sci. 2022. PMID: 34911657 Free PMC article. Review.
-
Current Challenges and Opportunities in Designing Protein-Protein Interaction Targeted Drugs.Adv Appl Bioinform Chem. 2020 Nov 12;13:11-25. doi: 10.2147/AABC.S235542. eCollection 2020. Adv Appl Bioinform Chem. 2020. PMID: 33209039 Free PMC article. Review.
-
Modulating Protein-Protein Interactions by Cyclic and Macrocyclic Peptides. Prominent Strategies and Examples.Molecules. 2021 Jan 16;26(2):445. doi: 10.3390/molecules26020445. Molecules. 2021. PMID: 33467010 Free PMC article. Review.
Cited by
-
Cyclic Peptides in Pipeline: What Future for These Great Molecules?Pharmaceuticals (Basel). 2023 Jul 12;16(7):996. doi: 10.3390/ph16070996. Pharmaceuticals (Basel). 2023. PMID: 37513908 Free PMC article. Review.
-
HELM-GPT: de novo macrocyclic peptide design using generative pre-trained transformer.Bioinformatics. 2024 Jun 3;40(6):btae364. doi: 10.1093/bioinformatics/btae364. Bioinformatics. 2024. PMID: 38867692 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

