Loss of AKAP12 aggravates rheumatoid arthritis-like symptoms and cardiac damage in collagen-induced arthritis mice
- PMID: 36464273
- PMCID: PMC10202715
- DOI: 10.1538/expanim.22-0103
Loss of AKAP12 aggravates rheumatoid arthritis-like symptoms and cardiac damage in collagen-induced arthritis mice
Abstract
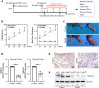
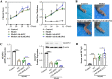
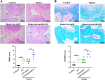
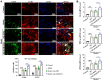
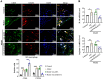
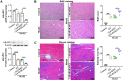
A-kinase anchoring protein 12 (AKAP12) has been identified as an anti-inflammatory and anti-fibrotic regulator in chronic inflammation and cardiovascular disease. However, the potential of AKAP12 in autoimmune disorders, rheumatoid arthritis (RA) and associated cardiac complications remains elusive. Here, a murine model of collagen-induced arthritis (CIA) was successfully induced, followed by adenovirus-mediated AKAP12 short hairpin RNA (shRNA) treatment. AKAP12 silenced mice displayed elevated clinical arthritis scores and significant ankle joint swelling. AKAP12 loss in CIA mice increased inflammatory cell infiltration and cartilage erosion, increased the levels of anti-IIC IgG and inflammatory cytokines IL-1β, IL-6, tumor necrosis factor (TNF)-α in serum, and upregulated the expression of cartilage-degrading enzymes MMP-1, MMP-3, MMP-13 in synovium, but reduced IL-10. The number of M1 macrophages and the expression of the markers (CCR7, IL-6, TNF-α and iNOS) was enhanced in synovial tissues, while M2 polarized macrophages and the makers (IL-10 and arginase-1) were reduced in response to AKAP12 loss. Moreover, low expression of AKAP12 was detected in the hearts of CIA mice. Loss of AKAP12 results in increased cardiac inflammation and fibrosis. This work suggests that AKAP12 loss aggravates joint inflammation likely through the promotion of M1 macrophage polarization and exacerbates inflammation-caused cardiac fibrosis.
Keywords: A-kinase anchoring protein 12 (AKAP12); heart; inflammation; macrophage polarization; rheumatoid arthritis.
Conflict of interest statement
The authors claim no conflict of interest to disclose.
Figures

Similar articles
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Wedelolactone ameliorates synovial inflammation and cardiac complications in a murine model of collagen-induced arthritis by inhibiting NF-κB/NLRP3 inflammasome activation.Folia Histochem Cytobiol. 2022;60(4):301-310. doi: 10.5603/FHC.a2022.0025. Epub 2022 Nov 16. Folia Histochem Cytobiol. 2022. PMID: 36380683
-
Wutou decoction attenuates the synovial inflammation of collagen-induced arthritis rats via regulating macrophage M1/M2 type polarization.J Ethnopharmacol. 2023 Jan 30;301:115802. doi: 10.1016/j.jep.2022.115802. Epub 2022 Oct 6. J Ethnopharmacol. 2023. PMID: 36209953
-
Pathogenesis of Collagen-Induced Arthritis: Role of Immune Cells with Associated Cytokines and Antibodies, Comparison with Rheumatoid Arthritis.Folia Biol (Praha). 2023;69(2):41-49. doi: 10.14712/fb2023069020041. Folia Biol (Praha). 2023. PMID: 38063000 Review.
-
Rheumatoid arthritis and interleukin-32.Cell Mol Life Sci. 2007 Oct;64(19-20):2671-9. doi: 10.1007/s00018-007-7186-8. Cell Mol Life Sci. 2007. PMID: 17619821 Free PMC article. Review.
Cited by
-
CGRP promotes osteogenic differentiation by regulating macrophage M2 polarization through HDAC6/AKAP12 signaling pathway.Regen Med. 2024;19(7-8):379-391. doi: 10.1080/17460751.2024.2370697. Epub 2024 Jul 29. Regen Med. 2024. PMID: 39072399
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

