A replication-deficient H9N2 influenza virus carrying H5 hemagglutinin conferred protection against H9N2 and H5N1 influenza viruses in mice
- PMID: 36458187
- PMCID: PMC9705590
- DOI: 10.3389/fmicb.2022.1042916
A replication-deficient H9N2 influenza virus carrying H5 hemagglutinin conferred protection against H9N2 and H5N1 influenza viruses in mice
Abstract
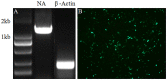
H5N1 and H9N2 influenza viruses have been reported to cause human infections and are believed to have pandemic potential. The vaccine is an effective tool to prevent influenza virus infection. However, inactivated influenza vaccines sometimes result in low antigenicity as result leads to generating of incomplete immune protection in the form of low cellular and humoral immunity. While the low temperature adapted, traditional live attenuated influenza vaccine (LAIV) is associated with the potential risk to revert to a virulent phenotype, there appears an essential need for an alternative potent methodology to design and develop influenza vaccines with substantial safety and efficacy which may confer solid protection against H9N2 or H5N1 influenza virus infections. In the present study, a replication-deficient recombinant influenza virus, WM01ma-HA(H5), expressing hemagglutinin (HA) of both H9N2 and H5N1 subtypes was developed. The chimeric gene segment expressing HA(H5), was designed using the sequence of an open reading frame (ORF) of HA adopted from A/wild duck/Hunan/021/2005(H5N1)(HN021ma) which was flanked by the NA packaging signals of mouse-adapted strain A/Mink/Shandong/WM01/2014(H9N2)(WM01ma). Due to the absence of ORF of structural protein NA, the replication of this engineered H9N2 influenza viruses WM01ma-HA(H5) was hampered in vitro and in vivo but was well competent in MDCK cells stably expressing the NA protein of WM01ma. Intranasal vaccination of mice with WM01ma-HA(H5) stimulated robust immune response without any clinical signs and conferred complete protection from infection by H5N1 or H9N2 subtype influenza viruses.
Keywords: H5N1; H9N2; influenza A virus; recombination; replication-deficient virus vaccine.
Copyright © 2022 Ren, Pei, Jiang, Zhao, Jiang, Liu, Yi, Hui and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A Replication-Defective Influenza Virus Harboring H5 and H7 Hemagglutinins Provides Protection against H5N1 and H7N9 Infection in Mice.J Virol. 2021 Jan 13;95(3):e02154-20. doi: 10.1128/JVI.02154-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177192 Free PMC article.
-
Vaccine Efficacy of Inactivated, Chimeric Hemagglutinin H9/H5N2 Avian Influenza Virus and Its Suitability for the Marker Vaccine Strategy.J Virol. 2017 Feb 28;91(6):e01693-16. doi: 10.1128/JVI.01693-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077631 Free PMC article.
-
Generation of a reassortant avian influenza virus H5N2 vaccine strain capable of protecting chickens against infection with Egyptian H5N1 and H9N2 viruses.Vaccine. 2016 Jan 4;34(2):218-224. doi: 10.1016/j.vaccine.2015.11.037. Epub 2015 Nov 25. Vaccine. 2016. PMID: 26620838 Free PMC article.
-
Development of a dual-protective live attenuated vaccine against H5N1 and H9N2 avian influenza viruses by modifying the NS1 gene.Arch Virol. 2015 Jul;160(7):1729-40. doi: 10.1007/s00705-015-2442-y. Epub 2015 May 12. Arch Virol. 2015. PMID: 25959557
-
Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt.Viruses. 2018 Mar 9;10(3):121. doi: 10.3390/v10030121. Viruses. 2018. PMID: 29522492 Free PMC article. Review.
Cited by
-
Pandemic preparedness through vaccine development for avian influenza viruses.Hum Vaccin Immunother. 2024 Dec 31;20(1):2347019. doi: 10.1080/21645515.2024.2347019. Epub 2024 May 28. Hum Vaccin Immunother. 2024. PMID: 38807261 Free PMC article. Review.
-
The Chinese Hamster Ovary Cell-Based H9 HA Subunit Avian Influenza Vaccine Provides Complete Protection against the H9N2 Virus Challenge in Chickens.Viruses. 2024 Jan 22;16(1):163. doi: 10.3390/v16010163. Viruses. 2024. PMID: 38275973 Free PMC article.
References
LinkOut - more resources
Full Text Sources

