Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system
- PMID: 36450835
- PMCID: PMC9712653
- DOI: 10.1038/s41598-022-24945-5
Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system
Abstract
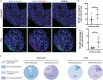
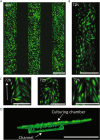
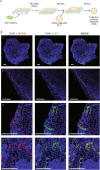
Kidney organoids derived from human induced pluripotent stem cells (iPSCs) have proven to be a valuable tool to study kidney development and disease. However, the lack of vascularization of these organoids often leads to insufficient oxygen and nutrient supply. Vascularization has previously been achieved by implantation into animal models, however, the vasculature arises largely from animal host tissue. Our aim is to transition from an in vivo implantation model towards an in vitro model that fulfils the advantages of vascularization whilst being fully human-cell derived. Our chip system supported culturing of kidney organoids, which presented nephron structures. We also showed that organoids cultured on chip showed increased maturation of endothelial populations based on a colocalization analysis of endothelial markers. Moreover, we observed migration and proliferation of human umbilical vein endothelial cells (HUVECs) cultured in the channels of the chip inside the organoid tissue, where these HUVECs interconnected with endogenous endothelial cells and formed structures presenting an open lumen resembling vessels. Our results establish for the first-time vascularization of kidney organoids in HUVEC co-culture conditions using a microfluidic organ-on-chip. Our model therefore provides a useful insight into kidney organoid vascularization in vitro and presents a tool for further studies of kidney development and drug testing, both for research purposes and pre-clinical applications.
© 2022. The Author(s).
Conflict of interest statement
C. Silvestri and N. Gaio and W. Quiros Solano are the founders of BIOND Solutions B.V. (BIOND). A. Othman is an employee of BIOND solutions B.V. All other authors declare no competing interests.
Figures

Similar articles
-
Efficient Vascularization of Kidney Organoids through Intracelomic Transplantation in Chicken Embryos.J Vis Exp. 2023 Feb 17;(192). doi: 10.3791/65090. J Vis Exp. 2023. PMID: 36876942
-
A perfusable, vascularized kidney organoid-on-chip model.Biofabrication. 2024 Jul 5;16(4). doi: 10.1088/1758-5090/ad5ac0. Biofabrication. 2024. PMID: 38906132
-
Maturation of Nephrons by Implanting hPSC-derived Kidney Progenitors Under Kidney Capsules of Unilaterally Nephrectomized Mice.Curr Stem Cell Res Ther. 2023;18(4):551-559. doi: 10.2174/1574888X17666220818101503. Curr Stem Cell Res Ther. 2023. PMID: 35984016
-
Strategies for Improving Vascularization in Kidney Organoids: A Review of Current Trends.Biology (Basel). 2023 Mar 26;12(4):503. doi: 10.3390/biology12040503. Biology (Basel). 2023. PMID: 37106704 Free PMC article. Review.
-
Bioengineering methods for vascularizing organoids.Cell Rep Methods. 2024 Jun 17;4(6):100779. doi: 10.1016/j.crmeth.2024.100779. Epub 2024 May 16. Cell Rep Methods. 2024. PMID: 38759654 Free PMC article. Review.
Cited by
-
Effective and new technologies in kidney tissue engineering.Front Bioeng Biotechnol. 2024 Oct 16;12:1476510. doi: 10.3389/fbioe.2024.1476510. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39479295 Free PMC article. Review.
-
Currently Used Methods to Evaluate the Efficacy of Therapeutic Drugs and Kidney Safety.Biomolecules. 2023 Oct 26;13(11):1581. doi: 10.3390/biom13111581. Biomolecules. 2023. PMID: 38002263 Free PMC article. Review.
-
Transformative Materials to Create 3D Functional Human Tissue Models In Vitro in a Reproducible Manner.Adv Healthc Mater. 2023 Aug;12(20):e2301030. doi: 10.1002/adhm.202301030. Epub 2023 Jun 13. Adv Healthc Mater. 2023. PMID: 37311209 Free PMC article.
-
Towards a New 3Rs Era in the construction of 3D cell culture models simulating tumor microenvironment.Front Oncol. 2023 Apr 3;13:1146477. doi: 10.3389/fonc.2023.1146477. eCollection 2023. Front Oncol. 2023. PMID: 37077835 Free PMC article. Review.
-
Endothelial cell provenance: an unclear role in transplant medicine.Front Transplant. 2023 Apr 28;2:1130941. doi: 10.3389/frtra.2023.1130941. eCollection 2023. Front Transplant. 2023. PMID: 38993867 Free PMC article. Review.
References
-
- Du Z, et al. Identification of predictive markers for the generation of well-differentiated human induced pluripotent stem cell-derived kidney organoids. Stem Cells Dev. 2021;30:1103–1114. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

