Amount of Pannexin 1 in Smooth Muscle Cells Regulates Sympathetic Nerve-Induced Vasoconstriction
- PMID: 36448464
- PMCID: PMC9851955
- DOI: 10.1161/HYPERTENSIONAHA.122.20280
Amount of Pannexin 1 in Smooth Muscle Cells Regulates Sympathetic Nerve-Induced Vasoconstriction
Abstract
Background: Panx1 (pannexin 1) forms high conductance channels that secrete ATP upon stimulation. The role of Panx1 in mediating constriction in response to direct sympathetic nerve stimulation is not known. Additionally, it is unknown how the expression level of Panx1 in smooth muscle cells (SMCs) influences α-adrenergic responses. We hypothesized that the amount of Panx1 in SMCs dictates the levels of sympathetic constriction and blood pressure.
Methods: To test this hypothesis, we used genetically modified mouse models enabling expression of Panx1 in vascular cells to be varied. Electrical field stimulation on isolated arteries and blood pressure were assessed.
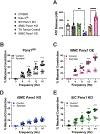
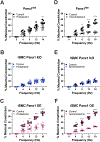
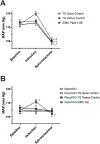
Results: Genetic deletion of SMC Panx1 prevented constriction by electric field stimulation of sympathetic nerves. Conversely, overexpression of Panx1 in SMCs using a ROSA26 transgenic model increased sympathetic nerve-mediated constriction. Connexin 43 hemichannel inhibitors did not alter constriction. Next, we evaluated the effects of altered SMC Panx1 expression on blood pressure. To do this, we created mice combining a global Panx1 deletion, with ROSA26-Panx1 under the control of an inducible SMC specific Cre (Myh11). This resulted in mice that could express only human Panx1, only in SMCs. After tamoxifen, these mice had increased blood pressure that was acutely decreased by the Panx1 inhibitor spironolactone. Control mice genetically devoid of Panx1 did not respond to spironolactone.
Conclusions: These data suggest Panx1 in SMCs could regulate the extent of sympathetic nerve constriction and blood pressure. The results also show the feasibility humanized Panx1-mouse models to test pharmacological candidates.
Keywords: blood pressure; pannexin; smooth muscle; spironolactone; sympathetic nerve.
Figures


Similar articles
-
Interaction Between Pannexin 1 and Caveolin-1 in Smooth Muscle Can Regulate Blood Pressure.Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):2065-2078. doi: 10.1161/ATVBAHA.118.311290. Arterioscler Thromb Vasc Biol. 2018. PMID: 30026274 Free PMC article.
-
Pannexin 1: a novel regulator of acute hypoxic pulmonary vasoconstriction.Cardiovasc Res. 2022 Aug 24;118(11):2535-2547. doi: 10.1093/cvr/cvab326. Cardiovasc Res. 2022. PMID: 34668529 Free PMC article.
-
A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells.Sci Signal. 2015 Feb 17;8(364):ra17. doi: 10.1126/scisignal.2005824. Sci Signal. 2015. PMID: 25690012 Free PMC article.
-
Sympathetic vasoconstriction takes an unexpected pannexin detour.Sci Signal. 2015 Feb 17;8(364):fs4. doi: 10.1126/scisignal.aaa7312. Sci Signal. 2015. PMID: 25690011 Review.
-
Emerging concepts regarding pannexin 1 in the vasculature.Biochem Soc Trans. 2015 Jun;43(3):495-501. doi: 10.1042/BST20150045. Biochem Soc Trans. 2015. PMID: 26009197 Free PMC article. Review.
Cited by
-
Connexin43, A Promising Target to Reduce Cardiac Arrhythmia Burden in Pulmonary Arterial Hypertension.Int J Mol Sci. 2024 Mar 14;25(6):3275. doi: 10.3390/ijms25063275. Int J Mol Sci. 2024. PMID: 38542257 Free PMC article. Review.
References
-
- Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E, Lorenz UM, Desai BN, Jaffe IZ, Bayliss DA, Isakson BE and Ravichandran KS. Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone. Circ Res. 2018;122:606–615. - PMC - PubMed
-
- Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA and Isakson BE. A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal. 2015;8:ra17. - PMC - PubMed
-
- DeLalio LJ, Keller AS, Chen J, Boyce AKJ, Artamonov MV, Askew-Page HR, Keller TCSt, Johnstone SR, Weaver RB, Good ME, Murphy SA, Best AK, Mintz EL, Penuela S, Greenwood IA, Machado RF, Somlyo AV, Swayne LA, Minshall RD and Isakson BE. Interaction Between Pannexin 1 and Caveolin-1 in Smooth Muscle Can Regulate Blood Pressure. Arterioscler Thromb Vasc Biol. 2018;38:2065–2078. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

