Long-Term and Low-Level Envelope C2V3 Stimulation by Highly Diverse Virus Isolates Leads to Frequent Development of Broad and Elite Antibody Neutralization in HIV-1-Infected Individuals
- PMID: 36445130
- PMCID: PMC9769935
- DOI: 10.1128/spectrum.01634-22
Long-Term and Low-Level Envelope C2V3 Stimulation by Highly Diverse Virus Isolates Leads to Frequent Development of Broad and Elite Antibody Neutralization in HIV-1-Infected Individuals
Abstract
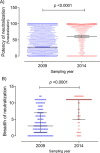
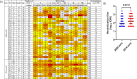
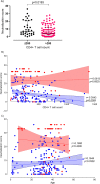
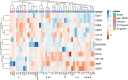
A minority of HIV-1-infected patients produce broadly neutralizing antibodies (bNAbs). Identification of viral and host correlates of bNAb production may help develop vaccines. We aimed to characterize the neutralizing response and viral and host-associated factors in Angola, which has one of the oldest, most dynamic, and most diverse HIV-1 epidemics in the world. Three hundred twenty-two HIV-1-infected adults from Angola were included in this retrospective study. Phylogenetic analysis of C2V3C3 env gene sequences was used for virus subtyping. Env-binding antibody reactivity was tested against polypeptides comprising the C2, V3, and C3 regions. Neutralizing-antibody responses were determined against a reference panel of tier 2 Env pseudoviruses in TZM-bl cells; neutralizing epitope specificities were predicted using ClustVis. All subtypes were found, along with untypeable strains and recombinant forms. Notably, 56% of the patients developed cross neutralizing, broadly neutralizing, or elite neutralizing responses. Broad and elite neutralization was associated with longer infection time, subtype C, lower CD4+ T cell counts, higher age, and higher titer of C2V3C3-specific antibodies relative to failure to develop bNAbs. Neutralizing antibodies targeted the V3-glycan supersite in most patients. V3 and C3 regions were significantly less variable in elite neutralizers than in weak neutralizers and nonneutralizers, suggesting an active role of V3C3-directed bNAbs in controlling HIV-1 replication and diversification. In conclusion, prolonged and low-level envelope V3C3 stimulation by highly diverse and ancestral HIV-1 isolates promotes the frequent elicitation of bNAbs. These results provide important clues for the development of an effective HIV-1 vaccine. IMPORTANCE Studies on neutralization by antibodies and their determinants in HIV-1-infected individuals have mostly been conducted in relatively recent epidemics caused by subtype B and C viruses. Results have suggested that elicitation of broadly neutralizing antibodies (bNAbs) is uncommon. The mechanisms underlying the elicitation of bNAbs are still largely unknown. We performed the first characterization of the plasma neutralizing response in a cohort of HIV-1-infected patients from Angola. Angola is characterized by an old and dynamic epidemic caused by highly diverse HIV-1 variants. Remarkably, more than half of the patients produced bNAbs, mostly targeting the V3-glycan supersite in HIV-1. This was associated with higher age, longer infection time, lower CD4+ T cell counts, subtype C infection, or higher titer of C2V3C3-specific antibodies relative to patients that did not develop bNAbs. These results may help develop the next generation of vaccine candidates for HIV-1.
Keywords: Angola; Env diversity; Env-specific antibodies; HIV-1 infection; bNAbs; broadly neutralizing antibodies; neutralizing epitopes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An HIV-1 Broadly Neutralizing Antibody from a Clade C-Infected Pediatric Elite Neutralizer Potently Neutralizes the Contemporaneous and Autologous Evolving Viruses.J Virol. 2019 Feb 5;93(4):e01495-18. doi: 10.1128/JVI.01495-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30429339 Free PMC article.
-
Subtle Longitudinal Alterations in Env Sequence Potentiate Differences in Sensitivity to Broadly Neutralizing Antibodies following Acute HIV-1 Subtype C Infection.J Virol. 2022 Dec 21;96(24):e0127022. doi: 10.1128/jvi.01270-22. Epub 2022 Dec 1. J Virol. 2022. PMID: 36453881 Free PMC article.
-
A Rare Mutation in an Infant-Derived HIV-1 Envelope Glycoprotein Alters Interprotomer Stability and Susceptibility to Broadly Neutralizing Antibodies Targeting the Trimer Apex.J Virol. 2020 Sep 15;94(19):e00814-20. doi: 10.1128/JVI.00814-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669335 Free PMC article.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
-
Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers.Retrovirology. 2018 Sep 5;15(1):61. doi: 10.1186/s12977-018-0443-0. Retrovirology. 2018. PMID: 30185183 Free PMC article. Review.
References
-
- Landais E, Huang X, Havenar-Daughton C, Murrell B, Price MA, Wickramasinghe L, Ramos A, Bian CB, Simek M, Allen S, Karita E, Kilembe W, Lakhi S, Inambao M, Kamali A, Sanders EJ, Anzala O, Edward V, Bekker LG, Tang J, Gilmour J, Kosakovsky-Pond SL, Phung P, Wrin T, Crotty S, Godzik A, Poignard P. 2016. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog 12:e1005369. doi:10.1371/journal.ppat.1005369. - DOI - PMC - PubMed
-
- Hu X, Hu Y, Zhao C, Gao H, Greene KM, Ren L, Ma L, Ruan Y, Sarzotti-Kelsoe M, Montefiori DC, Hong K, Shao Y. 2017. Profiling the neutralizing antibody response in chronically HIV-1 CRF07_BC-infected intravenous drug users naive to antiretroviral therapy. Sci Rep 7:46308. doi:10.1038/srep46308. - DOI - PMC - PubMed
-
- Rusert P, Kouyos RD, Kadelka C, Ebner H, Schanz M, Huber M, Braun DL, Hoze N, Scherrer A, Magnus C, Weber J, Uhr T, Cippa V, Thorball CW, Kuster H, Cavassini M, Bernasconi E, Hoffmann M, Calmy A, Battegay M, Rauch A, Yerly S, Aubert V, Klimkait T, Boni J, Fellay J, Regoes RR, Gunthard HF, Trkola A, Swiss HIV Cohort Study . 2016. Determinants of HIV-1 broadly neutralizing antibody induction. Nat Med 22:1260–1267. doi:10.1038/nm.4187. - DOI - PubMed
-
- Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 2014. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 88:12623–12643. doi:10.1128/JVI.01705-14. - DOI - PMC - PubMed
-
- Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Abdool Karim S, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L. 2011. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol 85:11502–11519. doi:10.1128/JVI.05363-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

