Ciclopirox drives growth arrest and autophagic cell death through STAT3 in gastric cancer cells
- PMID: 36443287
- PMCID: PMC9705325
- DOI: 10.1038/s41419-022-05456-7
Ciclopirox drives growth arrest and autophagic cell death through STAT3 in gastric cancer cells
Abstract
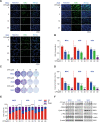
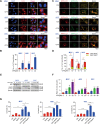
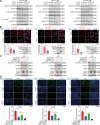
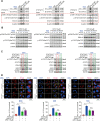
Ciclopirox (CPX), an antifungal drug, has recently been identified as a promising agent for cancer treatment. However, the effects and underlying mechanism of CPX as an antitumor agent of gastric cancer (GC) remain largely unknown. Here, we found that CPX dramatically suppresses GC xenograft growth in vitro via inhibiting proliferation and stimulating autophagic cell death rather than apoptosis. Moreover, CPX (20 mg/kg, intraperitoneally) substantially inhibits GC xenograft tumor growth in vivo. Mechanistically, CPX promotes growth arrest and autophagic cell death through suppressing the phosphorylation of signal transducers and activators of transcription 3 (STAT3) at tyrosine 705 (Tyr705) and serine 727 (Ser727) sites, respectively. Additionally, CPX induces STAT3 ubiquitination, which subsequently leads to a decrease in the p-STAT3 (Ser727) level. On the other hand, CPX represses the p-STAT3 (Tyr705) level via p-Src (Tyr416) inhibition. Collectively, our findings unmask a novel mechanism by which CPX regulates growth and autophagic cell death in GC cells via regulating the phosphorylation of STAT3 both at Tyr705 and Ser727 residues, and suggest that CPX may be a potential treatment for GC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Ciclopirox activates PERK-dependent endoplasmic reticulum stress to drive cell death in colorectal cancer.Cell Death Dis. 2020 Jul 27;11(7):582. doi: 10.1038/s41419-020-02779-1. Cell Death Dis. 2020. PMID: 32719342 Free PMC article.
-
Ciclopirox targets cellular bioenergetics and activates ER stress to induce apoptosis in non-small cell lung cancer cells.Cell Commun Signal. 2022 Mar 24;20(1):37. doi: 10.1186/s12964-022-00847-x. Cell Commun Signal. 2022. PMID: 35331268 Free PMC article.
-
Upregulation of tumor suppressor PIAS3 by Honokiol promotes tumor cell apoptosis via selective inhibition of STAT3 tyrosine 705 phosphorylation.J Nat Med. 2024 Mar;78(2):285-295. doi: 10.1007/s11418-023-01757-z. Epub 2023 Dec 11. J Nat Med. 2024. PMID: 38082192
-
Ciclopirox olamine inhibits mTORC1 signaling by activation of AMPK.Biochem Pharmacol. 2016 Sep 15;116:39-50. doi: 10.1016/j.bcp.2016.07.005. Epub 2016 Jul 7. Biochem Pharmacol. 2016. PMID: 27396756 Free PMC article.
-
Reposition of the Fungicide Ciclopirox for Cancer Treatment.Recent Pat Anticancer Drug Discov. 2021;16(2):122-135. doi: 10.2174/1574892816666210211090845. Recent Pat Anticancer Drug Discov. 2021. PMID: 33573561 Free PMC article. Review.
Cited by
-
Inhibition of mitochondrial OMA1 ameliorates osteosarcoma tumorigenesis.Cell Death Dis. 2024 Nov 1;15(11):786. doi: 10.1038/s41419-024-07127-1. Cell Death Dis. 2024. PMID: 39487118 Free PMC article.
-
Exploration of the link between COVID-19 and gastric cancer from the perspective of bioinformatics and systems biology.Front Med (Lausanne). 2024 Sep 20;11:1428973. doi: 10.3389/fmed.2024.1428973. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39371335 Free PMC article.
-
Discovery of potent STAT3 inhibitors using structure-based virtual screening, molecular dynamic simulation, and biological evaluation.Front Oncol. 2023 Nov 2;13:1287797. doi: 10.3389/fonc.2023.1287797. eCollection 2023. Front Oncol. 2023. PMID: 38023173 Free PMC article.
-
Carfilzomib activates ER stress and JNK/p38 MAPK signaling to promote apoptosis in hepatocellular carcinoma cells.Acta Biochim Biophys Sin (Shanghai). 2024 May 25;56(5):697-708. doi: 10.3724/abbs.2024040. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38591121 Free PMC article.
-
Identification and subsequent validation of transcriptomic signature associated with metabolic status in endometrial cancer.Sci Rep. 2023 Aug 23;13(1):13763. doi: 10.1038/s41598-023-40994-w. Sci Rep. 2023. PMID: 37612452 Free PMC article.
References
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung H, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

