Membrane-Active Cyclic Amphiphilic Peptides: Broad-Spectrum Antibacterial Activity Alone and in Combination with Antibiotics
- PMID: 36442155
- PMCID: PMC9743092
- DOI: 10.1021/acs.jmedchem.2c01469
Membrane-Active Cyclic Amphiphilic Peptides: Broad-Spectrum Antibacterial Activity Alone and in Combination with Antibiotics
Abstract
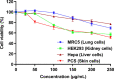
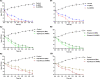
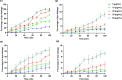
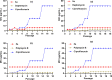
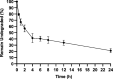
We designed a library of 24 cyclic peptides containing arginine (R) and tryptophan (W) residues in a sequential manner [RnWn] (n = 2-7) to study the impact of the hydrophilic/hydrophobic ratio, charge, and ring size on the antibacterial activity against Gram-positive and Gram-negative strains. Among peptides, 5a and 6a demonstrated the highest antimicrobial activity. In combination with 11 commercially available antibiotics, 5a and 6a showed remarkable synergism against a large panel of resistant pathogens. Hemolysis (HC50 = 340 μg/mL) and cell viability against mammalian cells demonstrated the selective lethal action of 5a against bacteria over mammalian cells. Calcein dye leakage and scanning electron microscopy studies revealed the membranolytic effect of 5a. Moreover, the stability in human plasma (t1/2 = 3 h) and the negligible ability of pathogens to develop resistance further reflect the potential of 5a for further development as a peptide-based antibiotic.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Broad-spectrum activity of membranolytic cationic macrocyclic peptides against multi-drug resistant bacteria and fungi.Eur J Pharm Sci. 2024 Jun 1;197:106776. doi: 10.1016/j.ejps.2024.106776. Epub 2024 Apr 23. Eur J Pharm Sci. 2024. PMID: 38663759
-
Small Amphiphilic Peptides: Activity Against a Broad Range of Drug-Resistant Bacteria and Structural Insight into Membranolytic Properties.J Med Chem. 2022 Jan 13;65(1):665-687. doi: 10.1021/acs.jmedchem.1c01782. Epub 2022 Jan 3. J Med Chem. 2022. PMID: 34978443
-
Design, Synthesis, and Evaluation of Amphiphilic Cyclic and Linear Peptides Composed of Hydrophobic and Positively-Charged Amino Acids as Antibacterial Agents.Molecules. 2018 Oct 22;23(10):2722. doi: 10.3390/molecules23102722. Molecules. 2018. PMID: 30360400 Free PMC article.
-
Cationic Amphiphilic Peptides: Synthetic Antimicrobial Agents Inspired by Nature.ChemMedChem. 2020 Oct 19;15(20):1887-1896. doi: 10.1002/cmdc.202000301. Epub 2020 Sep 8. ChemMedChem. 2020. PMID: 32767819 Review.
-
Antibiotics-Peptide Conjugates Against Multidrug-resistant Bacterial Pathogens.Curr Top Med Chem. 2018;18(22):1926-1936. doi: 10.2174/1568026619666181129141524. Curr Top Med Chem. 2018. PMID: 30499392 Review.
Cited by
-
Application of tobramycin benzyl ether as an antibiotic adjuvant capable of sensitizing multidrug-resistant Gram-negative bacteria to rifampicin.RSC Med Chem. 2024 Feb 16;15(3):1055-1065. doi: 10.1039/d3md00602f. eCollection 2024 Mar 20. RSC Med Chem. 2024. PMID: 38516601 Free PMC article.
-
Mechanistic Study of Antimicrobial Effectiveness of Cyclic Amphipathic Peptide [R4W4] against Methicillin-Resistant Staphylococcus aureus Clinical Isolates.Antibiotics (Basel). 2024 Jun 13;13(6):555. doi: 10.3390/antibiotics13060555. Antibiotics (Basel). 2024. PMID: 38927221 Free PMC article.
-
Structural Analysis and Activity Correlation of Amphiphilic Cyclic Antimicrobial Peptides Derived from the [W4R4] Scaffold.Molecules. 2023 Dec 12;28(24):8049. doi: 10.3390/molecules28248049. Molecules. 2023. PMID: 38138539 Free PMC article.
-
Design of a novel analogue peptide with potent antibiofilm activities against Staphylococcus aureus based upon a sapecin B-derived peptide.Sci Rep. 2024 Jan 26;14(1):2256. doi: 10.1038/s41598-024-52721-0. Sci Rep. 2024. PMID: 38278972 Free PMC article.
-
Effect of Camel Peptide on the Biofilm of Staphylococcus epidermidis and Staphylococcus haemolyticus Formed on Orthopedic Implants.Antibiotics (Basel). 2023 Nov 28;12(12):1671. doi: 10.3390/antibiotics12121671. Antibiotics (Basel). 2023. PMID: 38136705 Free PMC article.
References
-
- Moosazadeh Moghaddam M.; Aghamollaei H.; Kooshki H.; Azizi K.; Mirnejad R.; Choopani A. The development of antimicrobial peptides as an approach to prevention of antibiotic resistance. Rev. Med. Microbiol. 2015, 26, 98–110. 10.1097/MRM.0000000000000032. - DOI

