BHF177 Suppresses Diabetic Neuropathic Pain by Blocking PKC/CaMKII/ERK1/2/CREB Signaling Pathway through Activating GABAB Receptor
- PMID: 36439691
- PMCID: PMC9691330
- DOI: 10.1155/2022/4661519
BHF177 Suppresses Diabetic Neuropathic Pain by Blocking PKC/CaMKII/ERK1/2/CREB Signaling Pathway through Activating GABAB Receptor
Abstract
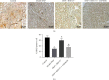
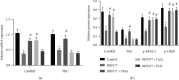
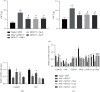
The gamma-aminobutyric acid type B (GABAB) receptor may participate in the development of diabetic neuropathic pain (DNP). BHF177 serves as a positive allosteric modulator of the GABAB receptor. In the current study, we sought to study the role of the BHF177-GABAB receptor in DNP and its underlying mechanism. Streptozotocin was adopted to induce a rat model of DNP, followed by determination of the paw withdrawal threshold (PWT), paw withdrawal latency (PWL), and glucose level. The effect of BHF177 on DNP by regulating the GABAB receptor in vivo was determined by the injection of BHF177 and/or CGP46381 (a GABAB receptor antagonist) into rat models of DNP. Hippocampal neuronal cells were isolated and cultured, and the neurons and DNP model rats were treated with activators of PKC (PMA), CaMKII (CaCl2), or ERK1/2 (EGF) to study the role of GABAB receptors in DNP via regulation of the NR2B-PKC-CaMKII-ERK-CREB pathway. BHF177 suppressed DNP symptoms by activating the GABAB receptors, as evidenced by increased PWT and PWL of DNP rats and the increased number of neurons expressing the GABAB receptor, but this effect was reversed by CGP46381 treatment. BHF177 treatment markedly repressed PKC, CaMKII, p-ERK1/2, and p-CREB expressions in the rat DNP model, but these suppressive effects were abrogated by treatments with PMA, CaCl2, or EGF treatment, respectively. To sum up, BHF177 suppresses DNP symptoms by blocking the PKC/CaMKII/ERK1/2/CREB signaling pathway to activate the GABAB receptors.
Copyright © 2022 Boyu Liu et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

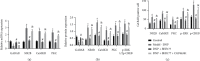

Similar articles
-
Effects of GABAB receptor positive allosteric modulator BHF177 and IRS-1 on apoptosis of hippocampal neurons in rats with refractory epilepsy via the PI3K/Akt pathway.Cell Biol Int. 2022 Nov;46(11):1775-1786. doi: 10.1002/cbin.11839. Epub 2022 Aug 21. Cell Biol Int. 2022. PMID: 35989486
-
Activation of GABAB Receptor Suppresses Diabetic Neuropathic Pain through Toll-Like Receptor 4 Signaling Pathway in the Spinal Dorsal Horn.Mediators Inflamm. 2018 Dec 10;2018:6016272. doi: 10.1155/2018/6016272. eCollection 2018. Mediators Inflamm. 2018. PMID: 30647535 Free PMC article.
-
Intrathecal baclofen, a GABAB receptor agonist, inhibits the expression of p-CREB and NR2B in the spinal dorsal horn in rats with diabetic neuropathic pain.Can J Physiol Pharmacol. 2014 Aug;92(8):655-60. doi: 10.1139/cjpp-2013-0463. Epub 2014 Jul 2. Can J Physiol Pharmacol. 2014. PMID: 24988216
-
Electroacupuncture Alleviates Streptozotocin-Induced Diabetic Neuropathic Pain via the TRPV1-Mediated CaMKII/CREB Pathway in Rats.J Mol Neurosci. 2024 Aug 20;74(3):79. doi: 10.1007/s12031-024-02256-w. J Mol Neurosci. 2024. PMID: 39162890
-
The role of protein kinases in diabetic neuropathic pain: an update review.J Diabetes Metab Disord. 2023 May 3;22(1):147-154. doi: 10.1007/s40200-023-01217-1. eCollection 2023 Jun. J Diabetes Metab Disord. 2023. PMID: 37255803 Free PMC article. Review.
References
-
- Zimmet P., Alberti K. G., Shaw J. Global and societal implications of the diabetes epidemic. Nature . 2001;414(6865):782–787. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

